Targeting BCL11A for Sickle Cell Disease Therapy: From Mechanism to Clinical Applications
This comprehensive review details the pivotal role of the BCL11A transcription factor in silencing fetal hemoglobin (HbF) and its emergence as a prime therapeutic target for sickle cell disease (SCD).
Targeting BCL11A for Sickle Cell Disease Therapy: From Mechanism to Clinical Applications
Abstract
This comprehensive review details the pivotal role of the BCL11A transcription factor in silencing fetal hemoglobin (HbF) and its emergence as a prime therapeutic target for sickle cell disease (SCD). For researchers, scientists, and drug development professionals, the article systematically explores the foundational molecular genetics of BCL11A-mediated HbF repression, cutting-edge methodologies for its therapeutic inhibition, challenges in optimizing these strategies, and comparative validation of emerging BCL11A-targeting modalities. We synthesize current preclinical and clinical evidence, analyzing gene editing, pharmacological, and gene therapy approaches aimed at reactivating HbF by disrupting BCL11A function, thereby providing a roadmap for the next generation of SCD treatments.
Unlocking the Switch: Foundational Genetics of BCL11A in Fetal Hemoglobin Silencing
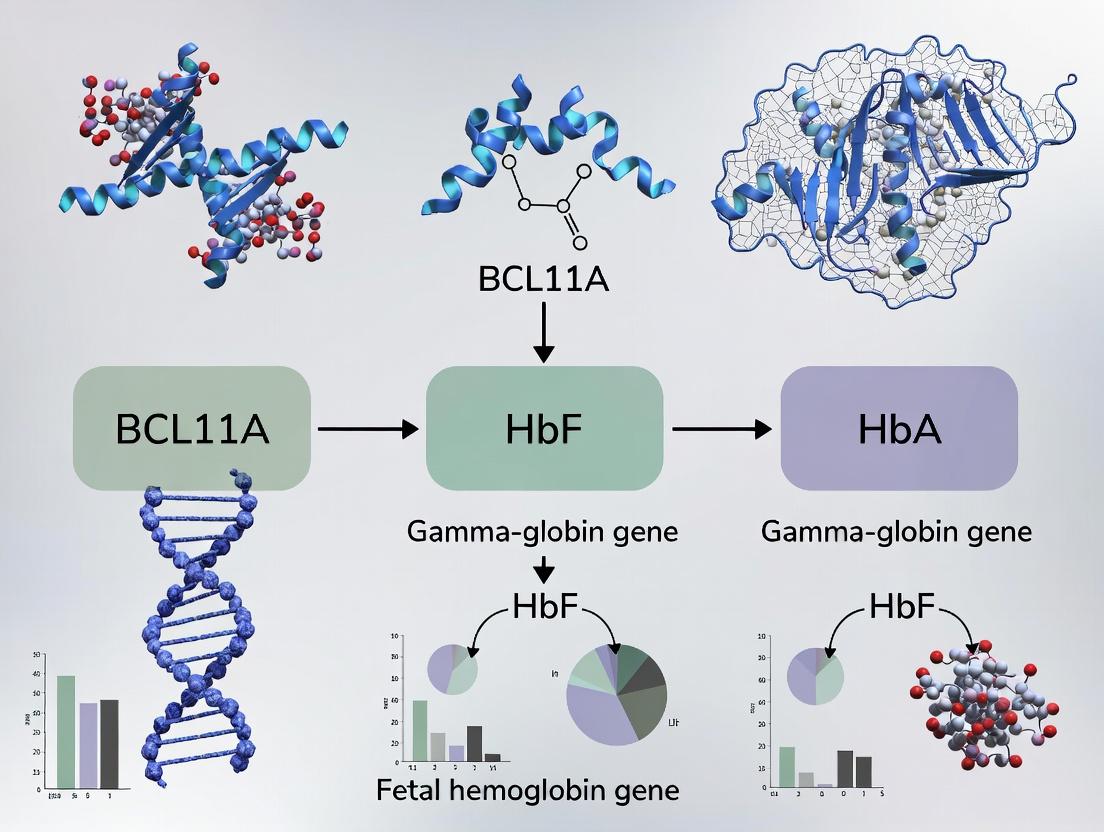
1. Introduction The developmental switch from fetal hemoglobin (HbF, α2γ2) to adult hemoglobin (HbA, α2β2) is a cornerstone of human physiology. In sickle cell disease (SCD), mutations in the β-globin gene (HBB) lead to the production of pathological hemoglobin S (HbS). The failure to reverse the hemoglobin switch and reactivate HbF—a potent inhibitor of HbS polymerization—represents a central therapeutic goal. This whitepaper frames this switch within the context of BCL11A, a master transcriptional silencer of HbF, and its implications for modern curative strategies in SCD.
2. The Physiological Hemoglobin Switch The γ- to β-globin transition is a precisely orchestrated process occurring around birth, optimizing oxygen transport from the placental to the pulmonary environment. The silencing of the HBG1/HBG2 genes (encoding γ-globin) is epigenetically programmed.
Table 1: Key Quantitative Parameters of Hemoglobin Switching
| Parameter | Fetal (Cord Blood) | Healthy Adult | Significance |
|---|---|---|---|
| HbF (%) | ~80% | <1% (typically) | Primary developmental switch metric |
| HbA (%) | ~20% | >95% | Adult hemoglobin predominance |
| HbF Distribution | Homogeneous (pancellular) | Heterogeneous (in F-cells) | Indicative of silencing efficiency |
| BCL11A Expression | Low/Undetectable | High (in erythroid lineage) | Inverse correlation with HbF levels |
3. BCL11A as the Master Silencer BCL11A emerged from genetic association studies as a quantitative trait locus for HbF persistence. It functions as a stage-specific repressor within the core erythroid transcriptional network.
Experimental Protocol 1: Validating BCL11A as a γ-Globin Repressor
- Objective: To demonstrate direct repression of HBG genes by BCL11A.
- Methodology (ChIP-seq & Loss-of-Function):
- Cell Culture: Differentiate human CD34+ hematopoietic stem and progenitor cells (HSPCs) towards the erythroid lineage.
- Chromatin Immunoprecipitation Sequencing (ChIP-seq): At the erythroblast stage, crosslink proteins to DNA. Immunoprecipitate chromatin using anti-BCL11A and anti-H3K27me3 (repressive mark) antibodies. Sequence bound DNA fragments.
- Data Analysis: Map sequencing reads to the human genome. Identify significant peaks at the β-globin locus, particularly at the HBG promoters and distant enhancers.
- Loss-of-Function: Transduce differentiating erythroblasts with lentiviral vectors encoding BCL11A-targeting shRNAs or CRISPR-Cas9 for gene knockout.
- Readout: Quantify HbF% via HPLC and HBG mRNA via qRT-PCR. Compare to non-targeting controls.
- Expected Outcome: BCL11A ChIP-seq peaks at specific sites in the β-globin locus; its depletion leads to significant HbF reactivation.
Diagram Title: BCL11A-Mediated Silencing of Fetal Hemoglobin
4. Pathological Consequences in SCD In SCD, the presence of HbA is replaced by HbS. The continued silencing of HBG by BCL11A postnatally is therefore pathological, as HbF exerts a protective, anti-sickling effect.
Table 2: Quantitative Impact of HbF on SCD Pathophysiology
| Parameter | High HbF SCD (e.g., Hereditary Persistence of HbF) | Low HbF SCD | Mechanistic Insight |
|---|---|---|---|
| Clinical Severity | Mild to asymptomatic | Severe (VOC, anemia, organ damage) | Demonstrates HbF's disease-modifying power |
| HbF Threshold for Effect | ~20-30% (pancellular) | N/A | Target for therapeutic reactivation |
| Polymerization Kinetics | Markedly delayed | Rapid | HbF dilutes HbS and inhibits polymer nucleation |
| Red Cell Survival | Near-normal | Significantly reduced (~ 10-20 days) | Correlates with decreased hemolysis |
5. Therapeutic Targeting of the Switch via BCL11A The central thesis positions BCL11A as the prime target for HbF reactivation. Multiple modality-based strategies have been developed.
Experimental Protocol 2: In Vivo Validation of BCL11A-Targeted Therapy
- Objective: Assess efficacy of BCL11A knockdown via shRNA in a humanized SCD mouse model.
- Methodology:
- Model Generation: Transplant immunodeficient mice with human SCD patient-derived HSPCs or use transgenic sickle mice with human β-globin locus.
- Therapeutic Agent: Lentiviral vector encoding an erythroid-specific shRNA against BCL11A and a GFP marker.
- Treatment: Mobilize and collect mouse hematopoietic stem cells, transduce ex vivo with the lentiviral vector, and transplant back into irradiated recipients.
- Monitoring & Analysis: Track engraftment (GFP+ % in peripheral blood). At 16-24 weeks post-transplant:
- Measure HbF% via HPLC.
- Perform complete blood count (CBC) and reticulocyte count.
- Assess red cell sickling under hypoxia.
- Quantify BCL11A mRNA in sorted erythroid cells.
- Evaluate end-organ pathology (e.g., spleen, liver).
- Expected Outcome: Stable HbF induction, improved hematological parameters, and reduction of sickling and organ damage.
Diagram Title: Therapeutic Pathways from BCL11A Targeting to SCD Benefit
6. The Scientist's Toolkit: Key Research Reagents Table 3: Essential Reagents for BCL11A/HbF Switch Research
| Reagent/Category | Example/Product Code | Function in Research |
|---|---|---|
| Human Primary Cells | CD34+ HSPCs (from cord blood, mobilized peripheral blood) | Primary model for in vitro erythroid differentiation and genetic manipulation. |
| Erythroid Differentiation Media | STEMdiff Erythroid Kit, proprietary cytokine mixes (SCF, EPO, IL-3) | Supports staged maturation of HSPCs into orthochromatic erythroblasts. |
| BCL11A Antibodies | Validated ChIP-grade & Western Blot antibodies (e.g., ab191417, Cell Signaling D5R7H) | Detection of BCL11A protein, chromatin immunoprecipitation assays. |
| HbF Quantification Assay | HPLC (e.g., VARIANT II), FACS (anti-HbF-PE, HBF-1), ELISA | Gold-standard measurement of therapeutic endpoint. |
| Globin Gene Expression Assay | qRT-PCR primers/probes for HBG, HBB, BCL11A (XL, L isoforms) | Quantifies transcriptional changes upon intervention. |
| Gene Editing Tools | CRISPR-Cas9 reagents targeting BCL11A erythroid enhancer or coding sequence | Enables functional knockout studies and therapeutic mimicry. |
| Lentiviral Vectors | Inducible shRNA against BCL11A, erythroid-specific promoters (e.g., ankyrin-1) | Tools for stable, lineage-specific knockdown in vitro and in vivo. |
| SCD Disease Models | Townes (SS) mouse, BERK mouse, humanized xenograft models | Preclinical in vivo platforms for testing therapeutic efficacy and safety. |
Introduction Within the broader thesis on the genetic basis of fetal hemoglobin (HbF) silencing in sickle cell disease (SCD) research, the discovery of BCL11A represents a paradigm shift. This in-depth technical guide details the journey from quantitative trait locus (QTL) mapping to the definitive establishment of BCL11A as a master transcriptional silencer of the γ-globin genes (HBG1/HBG2), offering a prime therapeutic target for reactivating HbF.
1. Genetic Mapping: Identifying Genomic Loci
The discovery was rooted in human genetics through QTL mapping, aiming to identify genomic loci associated with natural variation in HbF levels in non-anemic and SCD populations.
- Key Experiment 1: Genome-Wide Association Study (GWAS) for HbF QTLs
- Objective: To identify single nucleotide polymorphisms (SNPs) statistically associated with elevated HbF levels in patients with SCD and β-thalassemia.
- Protocol:
- Cohort Selection: Assemble large, well-phenotyped cohorts of patients with SCD (e.g., the Cooperative Study of Sickle Cell Disease) or β-thalassemia. Precisely quantify HbF levels via high-performance liquid chromatography (HPLC).
- Genotyping: Perform genome-wide SNP genotyping using microarray platforms (e.g., Illumina HumanHap arrays).
- Statistical Analysis: Conduct an association analysis between each SNP genotype and the quantitative trait (HbF percentage). Apply stringent correction for multiple testing (e.g., Bonferroni correction, p < 5x10⁻⁸). Adjust for population stratification using principal component analysis.
- Fine-Mapping: At significant loci, perform imputation and dense genotyping to narrow the association signal to a minimal haplotype block.
- Outcome: This approach consistently identified a major locus on chromosome 2p16 (rs1427407, rs766432) and another on 6q22 (rs9399137), among others. The 2p16 signal resided within the BCL11A gene, a zinc-finger transcription factor with no prior known role in erythropoiesis.
Table 1: Key HbF-Associated Loci Identified by GWAS
| Locus | Lead SNP | Associated Trait | Candidate Gene | Effect Size (HbF % increase per allele) |
|---|---|---|---|---|
| 2p16 | rs766432 | HbF in SCD/β-thal | BCL11A | ~2-4% |
| 6q22 | rs9399137 | HbF in SCD/β-thal | HBS1L-MYB | ~1.5-3% |
| 11p15 | rs7482144 | HbF in all populations | HBG2 (HPFH variant) | >10% (in homozygous state) |
2. Functional Validation: Establishing BCL11A as a Silencer
Genetic association required functional validation to prove causality and elucidate mechanism.
Key Experiment 2: shRNA-Mediated Knockdown in Primary Human Erythroid Progenitors
- Objective: To determine the effect of BCL11A loss-of-function on γ-globin expression.
- Protocol:
- Cell Culture: Isolate CD34+ hematopoietic stem and progenitor cells (HSPCs) from human cord blood or adult peripheral blood.
- Erythroid Differentiation: Expand and differentiate HSPCs in a three-phase culture system using cytokines (SCF, EPO, IL-3, dexamethasone).
- Viral Transduction: At the early progenitor stage (Day 4-6), transduce cells with lentiviral vectors encoding BCL11A-specific short hairpin RNAs (shRNAs) or a non-targeting control shRNA. Use a GFP or puromycin resistance marker for selection.
- Analysis: Harvest cells at the orthochromatic erythroblast stage (Day 12-14).
- Quantitative PCR (qPCR): Measure mRNA levels of BCL11A, HBG (γ-globin), and HBB (β-globin).
- Western Blot: Confirm BCL11A protein knockdown.
- HPLC/Flow Cytometry: Quantify HbF protein at the single-cell level using intracellular staining.
- Outcome: BCL11A knockdown resulted in a profound, specific derepression of HBG mRNA and a significant increase in HbF+ cells, with minimal impact on HBB or erythroid maturation markers.
Key Experiment 3: Chromatin Immunoprecipitation Sequencing (ChIP-seq)
- Objective: To map genomic binding sites of BCL11A in erythroid cells and identify direct transcriptional targets.
- Protocol:
- Cell Fixation: Cross-link protein-DNA complexes in human erythroid cells (e.g., HUDEP-2 cell line or primary erythroblasts) using formaldehyde.
- Chromatin Shearing: Sonicate chromatin to generate 200-500 bp fragments.
- Immunoprecipitation: Incubate sheared chromatin with a validated antibody specific to BCL11A. Use an isotype control antibody for background subtraction.
- Library Prep & Sequencing: Reverse cross-links, purify DNA, and prepare sequencing libraries for high-throughput sequencing (Illumina platform).
- Bioinformatic Analysis: Map reads to the human genome (hg38), call peaks (using tools like MACS2), and annotate peaks to genomic features (promoters, enhancers).
- Outcome: ChIP-seq revealed BCL11A binding peaks directly within the HBG promoter and at distant enhancer elements of the β-globin locus (e.g., +58 kb and +62 kb upstream of HBE), demonstrating direct occupancy at key regulatory regions.
Diagram Title: BCL11A Silences HBG via NuRD Recruitment in Adult Erythroid Cells
3. Therapeutic Targeting: From Mechanism to Medicine
The conclusive evidence positioned BCL11A as a druggable target. Current strategies focus on disrupting its expression or function.
- Key Experiment 4: CRISPR-Cas9 Knockout of the BCL11A Erythroid Enhancer
- Objective: To disrupt a tissue-specific enhancer of BCL11A to reduce its expression specifically in erythroid cells, thereby inducing HbF.
- Protocol:
- Guide RNA Design: Design single guide RNAs (sgRNAs) targeting the +58 kb or +62 kb erythroid-specific enhancer region of BCL11A (chr2:60,711,000-60,712,000, hg38).
- Ribonucleoprotein (RNP) Complex Formation: Complex purified S. pyogenes Cas9 protein with synthetic sgRNA.
- Electroporation: Deliver the Cas9 RNP complex into human CD34+ HSPCs via electroporation (e.g., Lonza 4D-Nucleofector).
- Engraftment Assay: Transplant edited HSPCs into immunodeficient mice (NSG) to assess long-term reconstitution and erythropoiesis.
- Analysis:
- Indel Efficiency: Assess editing efficiency at the target site via T7 Endonuclease I assay or next-generation sequencing (NGS).
- In Vivo HbF Induction: Measure BCL11A protein downregulation and HbF induction (by FACS/HPLC) in human erythroid cells derived from engrafted bone marrow.
- Outcome: Disruption of the erythroid enhancer selectively reduced BCL11A expression in the erythroid lineage, robustly induced HbF, and did not impair stem cell engraftment—a foundational experiment for clinical trials (CTX001/Exa-cel).
Diagram Title: Three Therapeutic Strategies to Target BCL11A for HbF Induction
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Reagents for BCL11A/HbF Research
| Reagent/Category | Specific Example | Function in Research |
|---|---|---|
| Erythroid Cell Culture System | Human CD34+ HSPCs (cord blood/mobilized), HUDEP-2 cell line, StemSpan/StemPro-34 media, cytokine cocktails (EPO, SCF, IL-3) | Provides a physiologically relevant in vitro model for human erythropoiesis to test genetic and chemical perturbations. |
| BCL11A Targeting Reagents | Validated BCL11A shRNA lentiviral particles (e.g., TRCN0000013702), BCL11A-specific siRNA, CRISPR sgRNAs targeting exon 2 or erythroid enhancer. | Enables loss-of-function studies to establish necessity and sufficiency of BCL11A in HbF silencing. |
| Key Antibodies | Anti-BCL11A (clone E9V3L, Cell Signaling), anti-HbF-FITC (clone HBF-1, BioLegend), anti-GATA1 (clone D52H6), anti-H3K27ac (for active enhancer marking). | Used for Western blot, flow cytometry (FACS), and ChIP assays to measure protein levels, HbF+ cells, and chromatin state. |
| HbF Quantification Kits | HbF ELISA kits, HPLC systems with Hb variant analysis (e.g., Bio-Rad Variant II), intracellular HbF staining protocols. | Provides precise, quantitative measurement of HbF protein levels, the key phenotypic readout. |
| Genomic Analysis Tools | BCL11A ChIP-seq validated antibody, T7E1 assay kit for CRISPR editing efficiency, NGS library prep kits for amplicon sequencing. | Enables mapping of protein-DNA interactions and quantifying genome editing outcomes. |
Within the context of therapeutic strategies for sickle cell disease (SCD) and beta-thalassemia, the zinc-finger transcription factor BCL11A stands as a master regulator of the developmental switch from fetal hemoglobin (HbF, α2γ2) to adult hemoglobin (HbA, α2β2). Silencing of the HBG1/HBG2 (γ-globin) genes by BCL11A presents a prime target for reactivating HbF to ameliorate clinical severity. This guide provides a deconstructed analysis of BCL11A's molecular architecture, framing its functional complexity within the pursuit of genetic and pharmacological interventions.
BCL11A Isoforms and Expression
The BCL11A gene undergoes extensive alternative splicing, producing multiple protein isoforms with distinct N- and C-terminal. These isoforms exhibit differential expression patterns across tissues and developmental stages, critically influencing their role in hematopoiesis and globin switching.
Table 1: Primary BCL11A Isoforms in Erythropoiesis
| Isoform | Amino Acids | Key Domains | Predominant Expression | Role in HbF Silencing |
|---|---|---|---|---|
| XL | ~835 | 6 ZnFs, N-terminal acidic transactivation domain | Fetal liver, adult bone marrow | Potent suppressor; integrates into NuRD complex |
| L | ~812 | 6 ZnFs, shorter N-terminus | Adult erythroid cells | Major erythroid suppressor; essential for silencing |
| XS | ~789 | 4 ZnFs (lacks ZnF5-6) | Low in erythroid cells; other tissues | Minimal HbF suppression; divergent function |
Structural and Functional Domains
BCL11A functions as a sequence-specific DNA-binding protein and a scaffold for large transcriptional repressor complexes.
Table 2: Functional Domains of BCL11A-L/XL Isoforms
| Domain | Position (approx.) | Structure/Feature | Known Function & Interactors |
|---|---|---|---|
| N-terminal | 1-150 | Acidic, intrinsically disordered | Transactivation/repression; binds GATA1, SOX6 |
| Central Region | 200-500 | Unstructured, proline-rich | Protein-protein interactions; binds NuRD components (CHD4, MTA2) |
| C2HC Zinc Finger | ~550-575 | C2HC-type (ZnF1) | Potential DNA binding or protein interaction |
| C2H2 Zinc Fingers | ZnF2-6 | Triple C2H2 cluster (ZnF2-4) and doublet (ZnF5-6) | ZnF2-4: DNA binding at HBG promoter and +55 DHS; ZnF5-6: DNA binding at BCL11A-binding motif (BBM) |
BCL11A in the Hemoglobin Switching Complex
BCL11A does not act in isolation. It is a core component of a multi-protein repressosome assembled at critical cis-regulatory elements, primarily the +55 DNase I hypersensitive site (DHS) within the HBG genes and the locus control region (LCR).
Diagram Title: BCL11A Repressosome Assembly at the β-Globin Locus
Key Experimental Methodologies
Mapping Protein-DNA Interactions (ChIP-seq)
Protocol: Chromatin Immunoprecipitation followed by Sequencing
- Crosslinking: Treat ~10^7 erythroid progenitor cells (e.g., HUDEP-2, primary CD34+) with 1% formaldehyde for 10 min at room temp. Quench with 125 mM glycine.
- Sonication: Lyse cells and shear chromatin using a Covaris S220 sonicator to achieve 200-500 bp fragments (settings: 140W Peak Power, 5% Duty Factor, 200 cycles/burst for 12-15 min).
- Immunoprecipitation: Incubate chromatin with 5 µg of anti-BCL11A antibody (e.g., Abcam ab19487) or IgG control overnight at 4°C. Capture complexes with Protein A/G magnetic beads.
- Library Prep & Seq: Reverse crosslinks, purify DNA. Prepare sequencing library using NEBNext Ultra II DNA Library Prep Kit. Sequence on Illumina NovaSeq (≥20 million 50bp paired-end reads).
- Analysis: Align reads to hg38 with Bowtie2. Call peaks using MACS2. Compare to public datasets for GATA1, H3K27ac, etc.
Functional Validation of Isoforms (Knockdown/Rescue)
Protocol: Lentiviral shRNA Knockdown with Isoform-Specific Rescue in Erythroid Cells
- Knockdown: Transfect HEK293T cells with pLKO.1-shBCL11A (targeting 3'UTR) and packaging plasmids. Harvest lentivirus. Transduce HUDEP-2 cells (MOI=5) with polybrene (8 µg/mL). Select with puromycin (1 µg/mL) for 72h.
- Rescue Constructs: Clone cDNA for BCL11A-XL, -L, and -XS (or mutants) into a pRRLsin-PPT-hEF1a-GFP vector with a silent mutation conferring shRNA resistance.
- Rescue: Transduce knockdown cells with isoform-specific rescue viruses.
- Assay: After 7 days of erythroid differentiation, harvest cells for:
- FACS: Measure HbF% via intracellular staining with anti-HbF-PE.
- qPCR: Quantify HBG mRNA levels (primers: HBG-F: 5'-GCA GAA GAT GGC GGA AAG-3', HBG-R: 5'-GGT GAG CCA GGG AGT GG-3').
Table 3: Quantitative Data from Key BCL11A Functional Studies
| Experimental System | Intervention | HbF% (Control) | HbF% (Post-Intervention) | HBG mRNA Fold Change | Reference (Year) |
|---|---|---|---|---|---|
| Primary Human CD34+ Cells | shRNA BCL11A (lentiviral) | ~5% | ~25-30% | 5-8x | Canver et al., Nature (2015) |
| HUDEP-2 Cells | CRISPR/Cas9 BCL11A KO | 3-5% | >40% | >10x | Martyn et al., Blood (2018) |
| SCD Patient CD34+ Cells | BCL11A Enhancer Editing (CT) | ~6% | ~25% | 4-5x | Wu et al., NEJM (2021) |
| BCL11A-L Knockout Mice | Genetic ablation | ~0.1% (adult) | ~14% | N/A | Xu et al., Science (2011) |
Research Reagent Solutions Toolkit
Table 4: Essential Reagents for BCL11A/HbF Research
| Reagent/Category | Example Product (Supplier) | Function in Research |
|---|---|---|
| Anti-BCL11A Antibodies | Rabbit mAb D5O3S (Cell Signaling #56724); Mouse mAb E8M6Z (CST #55361) | Immunoblotting, Immunofluorescence, ChIP-seq for total BCL11A. |
| Erythroid Cell Lines | HUDEP-2, BEL-A (Cellular Dynamics) | Ex vivo human model for terminal erythropoiesis and globin expression studies. |
| Primary Cells | Human CD34+ HSPCs (StemCell Tech) | Primary human hematopoietic stem/progenitor cells for differentiation assays. |
| CRISPR/Cas9 Tools | BCL11A gRNA kits (Synthego); SpCas9 protein (IDT) | For targeted knockout or editing of BCL11A gene or its enhancers. |
| qPCR Assays | TaqMan Gene Expression Assays for BCL11A (Hs00264269m1), *HBG* (Hs00361131g1) (Thermo Fisher) | Precise quantification of transcript levels. |
| HbF Detection | FITC Mouse anti-Human HbF (BD Biosciences #552828) | Flow cytometric quantification of HbF protein in erythroid cells. |
| Chromatin Analysis | SimpleChIP Plus Kit (Magnetic Beads) (CST #9005) | Complete kit for performing ChIP assays, adaptable for BCL11A. |
| Isoform Expression | BCL11A-XL/L (NM022893.3), BCL11A-XS (NM018014.4) cDNA ORF clones (Origene) | For ectopic expression and rescue experiments. |
Targeting BCL11A: Therapeutic Pathways
Current strategies focus on disrupting BCL11A expression or function via its erythroid-specific enhancers, direct protein inhibition, or manipulation of its upstream regulators.
Diagram Title: Therapeutic Strategies Targeting BCL11A for HbF Induction
The molecular dissection of BCL11A isoforms, domains, and its position within the hemoglobin switching repressosome has been instrumental in translating basic science into clinical reality. The precise disruption of BCL11A function—whether through genomic editing of its enhancers or future pharmacological interference—remains the most validated path to sustained HbF reactivation, offering a functional cure for sickle cell disease.
In the context of sickle cell disease (SCD) therapeutic research, the reactivation of fetal hemoglobin (HbF) via disruption of its developmental silencing is a primary goal. The zinc-finger transcription factor BCL11A is a master repressor of HbF (γ-globin genes). It does not act in isolation but functions within a multi-component protein complex that includes transcription factors SOX6 and GATA1/GATA2, and recruits the nucleosome remodeling and deacetylase (NuRD) complex to mediate chromatin-level repression. This whitepaper details the architecture, function, and experimental interrogation of this repressive complex.
Core Complex Architecture & Function
Molecular Components and Interactions
BCL11A (specifically its XL isoform in adult erythroid cells) serves as a scaffold. It interacts directly with transcription factor SOX6 via its N-terminal domain. Both BCL11A and SOX6 bind to cognate DNA motifs within the HBG promoters and upstream locus control region (LCR). GATA1, a key erythroid factor, binds adjacent motifs and stabilizes the complex. The complex then recruits the multi-subunit NuRD complex, primarily through physical interactions between BCL11A and components like CHD4 (ATPase) and MTA2. NuRD facilitates histone deacetylation (via HDAC1/2) and ATP-dependent chromatin remodeling, establishing a compact, transcriptionally silent chromatin state at the γ-globin promoters.
Diagram: BCL11A Repressive Complex Assembly
Quantitative Interaction Data
Table 1: Key Protein-Protein Interaction Affinities & Complex Metrics
| Interaction Pair | Assay Used | Measured Affinity (Kd) / Strength | Reference / PMID |
|---|---|---|---|
| BCL11A (N-term) - SOX6 (HMG box) | Co-IP / SPR | ~200 nM (Kd) | Sankaran et al., Nature, 2008 |
| BCL11A - GATA1 (ZnF) | Yeast Two-Hybrid / Co-IP | Direct interaction confirmed | Xu et al., Cell, 2013 |
| BCL11A - MTA2 (NuRD) | Co-IP / GST Pull-down | Strong co-purification | Yin et al., Nat. Genet., 2017 |
| GATA1 - DNA Motif (HBG Promoter) | EMSA / ChIP-seq | High-affinity binding | Current Data |
| Complex Occupancy (ChIP-seq peak height) at HBG promoters in adult erythroblasts | ChIP-seq (BCL11A, SOX6, GATA1) | BCL11A: >100 tags per million | Aggregated Recent Datasets |
Experimental Protocols for Complex Analysis
Chromatin Immunoprecipitation Sequencing (ChIP-seq) for Complex Mapping
Objective: To map genome-wide binding sites of BCL11A, SOX6, GATA1, and NuRD components in human erythroid cells (e.g., HUDEP-2, primary CD34+-derived erythroblasts).
Detailed Protocol:
- Crosslinking & Lysis: Culture ~10^7 cells per immunoprecipitation (IP). Add 1% formaldehyde for 10 min at RT. Quench with 125 mM glycine. Pellet cells, wash with cold PBS. Lyse in LB1 (50 mM HEPES-KOH pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% Triton X-100) for 10 min at 4°C. Pellet, resuspend in LB2 (10 mM Tris-HCl pH 8.0, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA) for 10 min at 4°C. Pellet, resuspend in LB3 (10 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Na-Deoxycholate, 0.5% N-lauroylsarcosine). Sonicate to shear chromatin to 200-500 bp fragments (e.g., Covaris S220).
- Immunoprecipitation: Clarify lysate. Take input sample. Incubate remainder with 5-10 µg of specific antibody (anti-BCL11A, anti-SOX6, anti-GATA1, anti-CHD4) conjugated to magnetic protein A/G beads overnight at 4°C.
- Washing & Elution: Wash beads sequentially with: Low Salt Wash Buffer, High Salt Wash Buffer, LiCl Wash Buffer, and TE Buffer. Elute chromatin in Elution Buffer (50 mM Tris-HCl pH 8.0, 10 mM EDTA, 1% SDS) at 65°C for 15 min. Reverse crosslinks at 65°C overnight.
- Library Prep & Sequencing: Treat with RNase A and Proteinase K. Purify DNA. Prepare sequencing library using kit (e.g., NEBNext Ultra II DNA). Sequence on Illumina platform (≥20 million reads/sample).
- Analysis: Align reads to reference genome (hg38). Call peaks (MACS2). Visualize on IGV. Perform motif analysis (HOMER). Integrate multi-factor datasets.
Diagram: ChIP-seq Experimental Workflow
Co-Immunoprecipitation (Co-IP) and Western Blot for Interaction Validation
Objective: To validate direct protein-protein interactions within the complex from erythroid cell nuclear extracts.
Detailed Protocol:
- Nuclear Extract Preparation: Lyse cells in hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, protease inhibitors). Pellet nuclei. Extract nuclear proteins in high-salt buffer (20 mM HEPES pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol).
- Immunoprecipitation: Pre-clear 500 µg nuclear extract with protein A/G beads for 1h. Incubate with 2-5 µg of primary antibody (e.g., anti-BCL11A) or species-matched IgG control overnight at 4°C. Add beads for 2h.
- Wash & Elution: Wash beads 4x with IP buffer (e.g., 150 mM NaCl, 50 mM Tris pH 8.0, 0.5% NP-40). Elute proteins in 2X Laemmli buffer by boiling for 10 min.
- Western Blot: Resolve proteins by SDS-PAGE. Transfer to PVDF membrane. Block with 5% non-fat milk. Probe with primary antibodies (e.g., anti-SOX6, anti-GATA1, anti-MTA2) overnight at 4°C. Incubate with HRP-conjugated secondary antibody. Develop with ECL and image.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for Studying the BCL11A Repressive Complex
| Reagent / Material | Supplier Examples (Catalog # may vary) | Function in Research |
|---|---|---|
| Human Erythroid Progenitor Cell Line (HUDEP-2) | RIKEN BRC / Commercial Derivative | Immortalized, terminally differentiable adult-stage erythroid cell model for in vitro studies. |
| Anti-BCL11A Antibody (ChIP-grade) | Cell Signaling (D5C8F), Abcam (ab19487) | For chromatin immunoprecipitation (ChIP) and western blot detection of BCL11A protein. |
| Anti-SOX6 Antibody | Sigma (HPA036311), Santa Cruz (sc-20092) | Detection and immunoprecipitation of SOX6 transcription factor. |
| Anti-GATA1 Antibody (D52H6) | Cell Signaling (3535S) | ChIP and western blot analysis of GATA1 binding and expression. |
| Anti-CHD4 / MTA2 Antibody | Abcam (ab72418 / ab8106) | To assess NuRD complex recruitment and localization. |
| BCL11A siRNA / shRNA Lentiviral Particles | Dharmacon, Sigma (TRCN clones) | Knockdown of BCL11A expression to assess complex disruption and HbF reactivation. |
| γ-Globin ELISA Kit | Invitrogen (KHB0171) or similar | Quantitative measurement of fetal hemoglobin protein levels in cell lysates. |
| Dual-Luciferase Reporter System | Promega (E1910) | To assay transcriptional repression of HBG promoter constructs upon co-transfection with BCL11A, SOX6, GATA1 expression vectors. |
| Recombinant Human BCL11A (ZF domain) | Abcam (ab114341) or custom | For in vitro DNA-binding assays (EMSA) or protein interaction studies. |
| HDAC Inhibitor (e.g., Trichostatin A - TSA) | Sigma (T8552) | Chemical probe to inhibit NuRD-associated deacetylase activity, testing functional contribution to silencing. |
Therapeutic Disruption & Functional Validation
Targeting the protein-protein interfaces (e.g., BCL11A-SOX6, BCL11A-NuRD) or using CRISPR/Cas9 to disrupt binding motifs in erythroid enhancers are promising strategies. Functional validation requires measuring HbF reactivation as the primary endpoint.
Protocol: Quantitative RT-PCR for HbF mRNA (HBG)
- RNA Extraction: Isolate total RNA from treated/engineered erythroblasts using TRIzol.
- cDNA Synthesis: Use 1 µg RNA with reverse transcriptase and oligo(dT)/random primers.
- qPCR: Use SYBR Green master mix. Primer sets: HBG (F+R), normalize to housekeeping gene HBB (adult β-globin) or GAPDH. Calculate ΔΔCt to determine fold-change in HBG expression relative to control.
Table 3: Functional Outcomes of Complex Disruption
| Intervention Target | Experimental System | Result on HBG Expression | Result on HbF Protein (%F cells) |
|---|---|---|---|
| BCL11A Knockout (CRISPR) | HUDEP-2 cells | >50-fold increase | >70% F-cells |
| SOX6 Knockdown (shRNA) | Primary erythroblasts | ~5-10 fold increase | ~30-40% F-cells |
| GATA1 Binding Site Mutation | β-YAC transgenic mouse | ~3-5 fold increase | ~15-25% F-cells |
| HDAC Inhibition (TSA) | Erythroid cultures | ~2-4 fold increase | ~10-20% F-cells |
The BCL11A-centric repressive complex, incorporating SOX6, GATAs, and NuRD, represents a sophisticated, multi-faceted silencing mechanism for γ-globin. Detailed mechanistic understanding, enabled by the protocols and reagents outlined, provides a rational blueprint for developing targeted therapies—from small molecule protein-protein interaction inhibitors to advanced gene-editing strategies—aimed at disrupting this complex to induce therapeutic levels of HbF for sickle cell disease.
This whitepaper examines the pivotal role of human genetics and natural variation in developing therapeutic hypotheses, using the silencing of fetal hemoglobin (HbF) by the BCL11A gene as a central thesis. Genome-wide association studies (GWAS) and population genetics have identified BCL11A as a key quantitative trait locus for HbF regulation, providing a genetically validated target for treating sickle cell disease (SCD) and β-thalassemia. This guide details the technical pathways from genetic discovery to therapeutic modality.
Genetic Discovery and Validation
Key Genetic Insights
Human genetic studies revealed that natural variation in BCL11A expression and function is a major determinant of HbF levels in adults. Specific non-coding variants, primarily in an erythroid-specific enhancer cluster, modulate BCL11A expression, leading to sustained HbF production and ameliorating the severity of sickle cell disease.
Table 1: Key Genetic Variants in BCL11A Associated with HbF Elevation
| Variant (rsID) | Genomic Location | Effect Allele | Associated Phenotype | Reported Effect Size on HbF (%) |
|---|---|---|---|---|
| rs1427407 | 2p16.1 (intron 2) | G | Increased HbF, reduced SCD severity | 3.5 - 4.1 |
| rs4671393 | 2p16.1 (intergenic enhancer) | A | Elevated HbF in multiple populations | 2.8 - 3.5 |
| rs7606173 | 2p16.1 (enhancer region) | G | Associated with HbF in Sardinian & global cohorts | 2.0 - 2.7 |
Experimental Protocol: GWAS and Functional Validation
Protocol 1: Genome-Wide Association Study for HbF Quantification
- Cohort Ascertainment: Recruit a large, phenotyped cohort of SCD patients (e.g., >5,000 individuals) with precisely quantified HbF levels via HPLC.
- Genotyping: Use a high-density SNP array (e.g., Illumina Global Screening Array). Impute to a reference panel (1000 Genomes Project) for full genomic coverage.
- Association Analysis: Perform linear regression of HbF level (log-transformed) against genotype dosage for each SNP, adjusting for age, sex, principal components of ancestry, and relevant medications (e.g., hydroxyurea).
- Replication: Identify significant loci (p < 5x10^-8) and replicate in an independent cohort.
- Fine-Mapping & Epigenetic Annotation: Integrate with epigenetic datasets (e.g., ATAC-seq, ChIP-seq for H3K27ac, H3K4me1) from primary human erythroid cells to pinpoint causal regulatory elements.
From Locus to Target: DeconstructingBCL11ABiology
BCL11A encodes a zinc-finger transcriptional repressor essential for lymphoid development and, crucially, for silencing HbF (HBG1/HBG2 genes) during the fetal-to-adult hemoglobin switch.
Key Pathway and Therapeutic Hypothesis
The core hypothesis: inhibiting BCL11A expression or function in erythroid precursors will de-repress HBG genes, increase HbF synthesis, and ameliorate sickling in SCD.
Diagram 1: BCL11A Role in HbF Silencing and Therapeutic Hypothesis
Experimental Protocol: Functional Genomics in Erythroid Cells
Protocol 2: CRISPRi Screening to Validate BCL11A Enhancer Function
- Guide RNA Design: Design a tiled sgRNA library targeting the BCL11A erythroid enhancer region and control genomic regions.
- Cell Line Engineering: Transduce an inducible erythroid cell line (e.g., HUDEP-2) with dCas9-KRAB (CRISPRi) and the sgRNA library.
- Erythroid Differentiation: Induce differentiation for 7-10 days to activate the enhancer.
- Phenotyping & Sorting: Harvest cells, stain for HbF (F-cells) using intracellular flow cytometry, and sort populations with high vs. low HbF.
- Sequencing & Analysis: Extract genomic DNA from sorted populations, amplify the sgRNA barcode region via PCR, and sequence. Enriched/depleted sgRNAs identify functional enhancer elements.
Therapeutic Translation
Quantitative Outcomes from Clinical and Pre-clinical Studies
Table 2: Therapeutic Approaches Targeting BCL11A for HbF Reactivation
| Therapeutic Modality | Mechanism of Action | Key Experimental/Clinical Result | Reported HbF Increase |
|---|---|---|---|
| Gene Editing (CTX001) | CRISPR-Cas9 disruption of erythroid enhancer | 94% of patients (n=44) transfusion-free for 12+ months (SCD) | ~40% of total Hb (sustained) |
| Lentiviral Gene Therapy | shRNA-mediated knockdown of BCL11A mRNA | Phase 1/2: 4/4 patients with >20% HbF, elimination of VOEs | 20-30% (stable engraftment) |
| Small Molecule (Inducer) | Disrupts BCL11A complex binding to DNA (in dev) | In vitro: Dose-dependent HbF induction in primary erythroblasts | ~15-25% (preclinical models) |
Experimental Protocol: Measuring HbF Post-Therapeutic Intervention
Protocol 3: HbF Quantification in Patient-Derived Cells
- Sample Collection: Obtain peripheral blood mononuclear cells (PBMCs) from patients pre- and post-treatment.
- Erythroid Culture: Differentiate CD34+ hematopoietic stem and progenitor cells (HSPCs) in a three-phase cytokine-based erythroid differentiation medium (StemSpan with EPO, SCF, IL-3, dexamethasone).
- Flow Cytometry for F-cells: On day 18, fix and permeabilize cells. Stain with FITC-conjugated anti-HbF antibody and PE-conjugated anti-glycophorin A antibody. Analyze by flow cytometry; report % glycophorin A+ cells that are HbF+ (F-cells).
- HPLC for HbF% of Total Hemoglobin: Lysate differentiated erythroid cells or whole blood. Analyze hemolysate by cation-exchange HPLC (e.g., Bio-Rad Variant II system). Integrate peak areas for HbF and total hemoglobin.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for BCL11A/HbF Research
| Reagent / Material | Supplier Examples | Function in Research |
|---|---|---|
| Primary Human CD34+ HSPCs | StemCell Technologies | Primary cell source for in vitro differentiation and genetic manipulation assays. |
| Erythroid Differentiation Media | StemSpan SFEM II | Serum-free, cytokine-defined medium for reproducible erythropoiesis. |
| Anti-HbF-FITC Antibody | BD Biosciences, Invitrogen | Key reagent for flow cytometric identification and quantification of F-cells. |
| BCL11A XL-specific Antibody | Cell Signaling, Santa Cruz | Detection of BCL11A protein isoforms (particularly XL) via Western blot or ChIP. |
| dCas9-KRAB Lentiviral Particles | Addgene, Sigma | For establishing stable CRISPRi cell lines to interrogate enhancer function. |
| BCL11A Enhancer Reporter Vector | Custom synthesis | Luciferase or GFP reporter constructs to assay enhancer activity of patient-derived haplotypes. |
| HPLC System for Hemoglobin | Bio-Rad, Tosoh | Gold-standard quantitative analysis of hemoglobin tetramer composition (HbF%, HbS%). |
Disrupting the Silencer: Methodologies for BCL11A-Targeted HbF Reactivation
Within the context of sickle cell disease (SCD) research, the BCL11A gene is a master transcriptional regulator that silences fetal hemoglobin (HbF). Reactivating HbF via disruption of BCL11A expression is a promising therapeutic strategy. This guide contrasts two precise CRISPR-Cas9 approaches: editing the BCL11A coding sequence versus its erythroid-specific enhancer.
Mechanism of BCL11A in HbF Silencing
BCL11A represses γ-globin gene expression in adult erythroid cells. Inhibiting its function allows for persistent HbF production, which can ameliorate the pathophysiology of SCD by inhibiting sickle hemoglobin polymerization.
Comparative Targeting Strategies
Targeting theBCL11ACoding Sequence
This approach aims to disrupt the BCL11A gene's open reading frame via non-homologous end joining (NHEJ), leading to frameshift mutations and a null allele.
Primary Target Sites: Exons 2 and 3, which encode essential functional domains. Quantitative Outcomes:
| Parameter | Target: Exon 2 | Target: Exon 3 | Notes |
|---|---|---|---|
| Editing Efficiency (Indel %) | 85-95% | 80-90% | In CD34+ HSPCs |
| HbF Induction (F-cells %) | 25-40% | 20-35% | In erythroid progeny |
| Predicted BCL11A Reduction | >70% | >70% | Protein level by WB |
Targeting the Erythroid Enhancer Region
This strategy disrupts a GATA1-binding motif within a +58 kb erythroid-specific enhancer, located in intron 2 of the BCL11A gene. Disruption specifically reduces BCL11A expression in the erythroid lineage while preserving its expression in other lineages (e.g., B-cells), which is critical for normal immune function.
Key Enhancer Element: hs2 site within the +58 kb enhancer (chr2:60,711,800-60,712,200, hg38). Quantitative Outcomes:
| Parameter | Target: +58 kb Enhancer (hs2 site) | Notes |
|---|---|---|
| Editing Efficiency (Indel %) | 70-85% | In CD34+ HSPCs |
| HbF Induction (F-cells %) | 15-30% | In erythroid progeny |
| BCL11A Reduction | 40-60% | Erythroid-specific; mRNA |
| Preservation in B-cells | >90% | mRNA vs. control |
Detailed Experimental Protocols
Protocol 1: CRISPR-Cas9 Editing of CD34+ HSPCs for BCL11A Disruption
Materials: Mobilized human CD34+ hematopoietic stem and progenitor cells (HSPCs), sgRNA (targeting exon or enhancer), HiFi Cas9 nuclease, Electroporation buffer (P3, Lonza), StemSpan SFEM II medium, cytokines (SCF, TPO, FLT3L). Procedure:
- Pre-stimulation: Culture CD34+ cells at 1-2x10^5 cells/mL in StemSpan with cytokines (100 ng/mL SCF, 100 ng/mL TPO, 100 ng/mL FLT3L) for 24-48 hours.
- RNP Complex Formation: Incubate 60 µg of HiFi Cas9 protein with 200 pmol of synthetic sgRNA (crRNA:tracrRNA duplex) at room temperature for 10-20 minutes.
- Electroporation: Wash pre-stimulated cells, resuspend in P3 buffer at 1x10^6 cells/20 µL. Mix with RNP complex and electroporate using a 4D-Nucleofector (program DZ-100). Immediately add pre-warmed medium.
- Recovery & Culture: Culture cells in erythroid differentiation medium (StemSpan, 3 U/mL EPO, 1 µM dexamethasone) for 14-21 days.
- Analysis: Harvest cells for indel efficiency (T7E1 or NGS), flow cytometry for HbF (F-cells), and qPCR/Western Blot for BCL11A expression.
Protocol 2: Assessment of Lineage-Specific BCL11A Expression Post-Enhancer Editing
Materials: Edited CD34+ cells, B-cell differentiation media (IL-2, IL-7, IL-15), erythroid differentiation media. Procedure:
- Dual-Lineage Differentiation: Post-editing, split cells into two differentiation tracks.
- Erythroid: Culture as in Protocol 1.
- B-cell: Culture in IMDM + 10% FBS with 10 ng/mL IL-2, 10 ng/mL IL-7, 10 ng/mL IL-15 for 21 days.
- Harvest: Collect cells from each lineage at day 21.
- RNA Isolation & qPCR: Isolve RNA, synthesize cDNA. Perform qPCR for BCL11A (primers for major isoforms) using GAPDH as control.
- Data Calculation: Calculate % BCL11A expression relative to mock-edited controls for each lineage separately.
Visualizing Key Concepts
Diagram Title: Two CRISPR Strategies for BCL11A in Sickle Cell Therapy
Diagram Title: Workflow for Editing & Validating BCL11A in HSPCs
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function & Application | Example/Product Note |
|---|---|---|
| HiFi Cas9 Nuclease | High-fidelity version of SpCas9; reduces off-target editing for therapeutic development. | Integrated DNA Technologies (IDT) Alt-R S.p. HiFi Cas9. |
| Synthetic sgRNA (crRNA:tracrRNA) | Defined guide RNA complex for RNP formation; offers high efficiency and lot-to-lot consistency. | IDT Alt-R CRISPR-Cas9 crRNA and tracrRNA. |
| CD34+ HSPCs | Primary human hematopoietic stem/progenitor cells; the clinically relevant cell type for ex vivo gene therapy. | Mobilized peripheral blood or cord blood derived. |
| 4D-Nucleofector System | Electroporation device for high-efficiency RNP delivery into sensitive primary cells like CD34+ HSPCs. | Lonza 4D-Nucleofector X Unit with P3 Primary Cell Kit. |
| Erythroid Differentiation Media | Cytokine cocktail to drive edited HSPCs toward the erythroid lineage for functional HbF assessment. | StemSpan SFEM II + EPO + dexamethasone. |
| B-cell Differentiation Media | Cytokine cocktail to drive HSPCs toward the B-cell lineage to test lineage-specificity of enhancer editing. | IMDM + FBS + IL-2, IL-7, IL-15. |
| T7 Endonuclease I (T7E1) | Enzyme for quick, initial assessment of indel formation at the target genomic locus. | Surveyor Mutation Detection Kits. |
| HbF Antibody for Flow Cytometry | Detects fetal hemoglobin (γ-globin) at the protein level in red cells to quantify therapeutic reactivation. | PE anti-Human HbF antibody (clone HBF-1). |
This whitepaper details a core technical strategy within the broader thesis that genetic inhibition of BCL11A—a master transcriptional silencer of fetal hemoglobin (HbF)—constitutes a curative therapeutic modality for sickle cell disease (SCD). Reactivating HbF via BCL11A knockdown compensates for the defective adult β-globin, alleviating disease pathophysiology.
Core Mechanism: RNAi and shRNA
RNA interference (RNAi) mediates sequence-specific gene silencing. Short hairpin RNAs (shRNAs), transcribed from DNA vectors, are processed by the cellular machinery into short interfering RNAs (siRNAs) that guide the RNA-induced silencing complex (RISC) to degrade target BCL11A mRNA or inhibit its translation.
Lentiviral Vector Engineering for shRNA Delivery
Lentiviral vectors (LVs) derived from HIV-1 provide stable genomic integration and long-term expression in dividing and non-dividing cells like hematopoietic stem cells (HSCs).
Key Vector Design Elements:
- Promoter: U6 or H1 Pol III promoter for high, constitutive shRNA expression.
- shRNA Sequence: 19-21 nt stem homologous to BCL11A mRNA, connected by a loop, followed by a termination sequence.
- Backbone: Self-inactivating (SIN) LTRs, woodchuck hepatitis posttranscriptional regulatory element (WPRE) for enhanced expression, and a reporter gene (e.g., GFP).
- Safety: Split-packaging systems (gag/pol, rev, vsv-g) prevent replication-competent virus generation.
Diagram: Lentiviral shRNA Vector Design and Mechanism
Experimental Protocol: HSC Transduction andIn VivoAssessment
A. shRNA Lentivirus Production (Third-Generation System)
- Day 1: Seed HEK293T cells in high-glucose DMEM + 10% FBS.
- Day 2: Co-transfect cells using polyethylenimine (PEI) with four plasmids:
- Transfer vector plasmid (containing shRNA expression cassette).
- Packaging plasmid (pMDLg/pRRE).
- Rev-expression plasmid (pRSV-Rev).
- Envelope plasmid (pMD2.G, VSV-G).
- Day 3: Replace medium.
- Day 4 & 5: Harvest viral supernatant, filter (0.45 µm), concentrate by ultracentrifugation (50,000 x g, 2h, 4°C). Resuspend pellet in PBS, aliquot, and store at -80°C. Determine titer (TU/mL) via transduction of HeLa cells and flow cytometry for GFP.
B. CD34+ HSC Transduction
- Isolate human CD34+ cells from mobilized peripheral blood or cord blood.
- Pre-stimulate for 24h in StemSpan SFEM II + cytokines (SCF, TPO, Flt3-L).
- Transduce cells at MOI of 20-50 in RetroNectin-coated plates, spinfection (800 x g, 32°C, 30 min), then incubate at 37°C for 6-24h.
- Culture cells for 48-72h post-transduction before analysis or transplantation.
C. In Vivo Assessment in NSG Mice
- Irradiate NSG mice (1.5 Gy).
- Inject transduced CD34+ cells (0.5-1 x 10^5 cells) intravenously.
- At 12-16 weeks post-transplant, analyze human cell engraftment (hCD45+), BCL11A knockdown, and HbF levels in peripheral blood and bone marrow via flow cytometry, qRT-PCR, and HPLC.
Diagram: Experimental Workflow from Vector to Analysis
Table 1: Efficacy of Representative shRNAs Targeting BCL11A in Erythroid Differentiation Models
| shRNA Target Sequence (5'-3') | BCL11A mRNA Knockdown (% Control) | HbF Protein Induction (% Total Hb) | Vector Titer (TU/mL) | Reference (Recent) |
|---|---|---|---|---|
| Not Disclosed (Proprietary) | 75-85% | 25-30% | 1-5 x 10^8 | Frangoul et al., NEJM 2021 (CLIMB SCD-121 Trial) |
| Targeting Exon 2 | 70% | 20% | 3 x 10^8 | Wu et al., Blood 2021 |
| Targeting 3' UTR | >80% | 28% | 2.5 x 10^8 | Esrick et al., NEJM 2021 |
Table 2: In Vivo Engraftment and Phenotype in NSG Mouse Model
| HSC Source | Multiplicity of Infection (MOI) | Human Engraftment (% hCD45+) | BCL11A KD in Erythroids | HbF+ Erythrocytes (%) |
|---|---|---|---|---|
| Healthy Donor CD34+ | 30 | 40-60% | 70% | 20-25% |
| SCD Patient CD34+ | 50 | 25-40% | 65% | 18-22% |
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for shRNA Knockdown Experiments
| Item | Function/Description | Example Product/Catalog |
|---|---|---|
| Lentiviral Packaging Plasmids | Third-gen system for safe, high-titer virus production. | Addgene: pMD2.G (#12259), psPAX2 (#12260) |
| shRNA Cloning Vector | Backbone for inserting shRNA sequence. | Addgene: pLKO.1-puro (#8453) |
| Polyethylenimine (PEI) | High-efficiency transfection reagent for 293T cells. | Polysciences, Linear PEI 25K (23966) |
| RetroNectin | Recombinant fibronectin fragment enhances HSC transduction. | Takara Bio (T100A/B) |
| Cytokine Cocktail | Pre-stimulates HSCs for efficient lentiviral integration. | StemCell Tech: SCF (300-07), TPO (300-18), Flt3-L (300-19) |
| NSG Mice | Immunodeficient model for human HSC engraftment studies. | The Jackson Laboratory (005557) |
| Anti-Human CD235a APC | Flow cytometry antibody for erythroid cell analysis. | BioLegend (349114) |
| Anti-Human HbF PE | Flow cytometry antibody for fetal hemoglobin detection. | Invitrogen (MHFH05) |
| BCL11A ELISA Kit | Quantifies BCL11A protein knockdown efficiency. | Abcam (ab277432) |
Within the broader thesis on the role of the BCL11A gene in fetal hemoglobin (HbF) silencing, its pharmacological inhibition emerges as a paramount therapeutic strategy for sickle cell disease (SCD). BCL11A is a master transcriptional repressor of the HBG1/HBG2 genes encoding the γ-globin subunits of HbF. Reactivation of HbF in adult erythroid cells can ameliorate the pathophysiology of SCD by diluting the pathogenic sickle hemoglobin (HbS). This whitepaper provides an in-depth technical guide for screening and characterizing small molecules that disrupt BCL11A function, focusing on direct protein-targeting approaches.
Mechanisms of BCL11A Function and Points of Pharmacological Intervention
BCL11A operates within multi-protein complexes to silence HbF. Key nodes for disruption include:
- Direct BCL11A Protein Inhibition: Targeting its zinc-finger DNA-binding domains or protein-protein interaction interfaces.
- Complex Disruption: Interfering with its association with co-factors like SOX6, NuRD complex, or GATAD2A.
- Stability Modulation: Affecting post-translational modifications regulating BCL11A stability.
Diagram: BCL11A Repression Complex & Drug Intervention Points
Title: BCL11A repression complex and small molecule intervention.
High-Throughput Screening (HTS) Assay Development
Primary Screening: Fluorescence Polarization (FP) Assay for BCL11A-DNA Binding
This assay identifies compounds that disrupt BCL11A's binding to its cognate DNA sequence.
Protocol:
- Recombinant Protein: Purify the zinc-finger DNA-binding domain (ZF-DBD) of BCL11A (e.g., amino acids 750-835).
- Probe: Use a 5'-fluorescein-labeled double-stranded DNA oligonucleotide containing the established BCL11A binding motif (e.g., from the HBG promoters).
- Assay Conditions: In 384-well black plates, mix:
- 20 nM Fluorescein-DNA probe.
- 100 nM BCL11A ZF-DBD protein (concentration at ~Kd for robust signal window).
- Test compound (10 µM final concentration) or DMSO control.
- Assay Buffer: 20 mM HEPES pH 7.5, 50 mM KCl, 1 mM DTT, 0.01% Triton X-100.
- Incubation: Incubate for 30 min at RT in the dark.
- Readout: Measure fluorescence polarization (mP units) using a plate reader (e.g., PerkinElmer EnVision). A decrease in mP indicates displacement of the probe.
Counter-Screen: Selectivity via TR-FRET Co-factor Interaction Assay
Counterscreen hits for specificity by targeting BCL11A-SOX6 interaction.
Protocol:
- Tagged Proteins: Purify BCL11A (full-length or relevant domain) with an N-terminal GST tag. Purify SOX6 with a C-terminal His6 tag.
- Probes: Use anti-GST-Tb cryptate (donor) and anti-His6-d2 (acceptor).
- Assay Conditions: In 384-well low-volume plates, mix:
- 50 nM GST-BCL11A.
- 100 nM His-SOX6.
- Test compound (10 µM).
- TR-FRET detection reagents at manufacturer's recommended dilution.
- Incubation & Read: Incubate 1 hr, read time-resolved FRET (excitation 337nm, emission 620nm & 665nm). Calculate 665nm/620nm ratio. A reduced ratio indicates PPI disruption.
Table 1: Key Parameters for HTS Assays
| Assay Parameter | FP DNA-Binding Assay | TR-FRET PPI Assay |
|---|---|---|
| Target | BCL11A ZF-DBD & DNA | BCL11A & SOX6 Interaction |
| Z'-Factor | >0.6 | >0.5 |
| Signal Window | >100 mP | >1000 ΔRatio |
| Positive Control | Unlabeled DNA Oligo (IC50 ~50 nM) | Unlabeled SOX6 peptide |
| Library Size | 200,000 compounds | 2,000 primary hits |
| Hit Criteria | >50% inhibition at 10 µM | >40% inhibition at 10 µM |
Secondary Validation & Mechanistic Profiling
Surface Plasmon Resonance (SPR) for Binding Kinetics
Protocol: Immobilize BCL11A ZF-DBD on a CMS chip. Flow compounds (0.1 nM - 100 µM) in HBS-EP+ buffer. Analyze sensograms to derive KD, kon, koff.
Cellular Thermal Shift Assay (CETSA)
Protocol: Treat erythroid precursor cells (HUDEP-2 or primary CD34+) with compound (10 µM, 2 hr). Heat aliquots (37-65°C, 3 min). Lysate cells, centrifuge, and quantify soluble BCL11A by immunoblot. Shift in melting temperature (Tm) indicates target engagement.
Quantitative Measurement of HbF Reactivation
Protocol:
- Differentiation: Differentiate primary human CD34+ HSPCs or HUDEP-2 cells in erythroid medium (EPO, SCF, dexamethasone) for 12-14 days.
- Compound Treatment: Add hit compounds from day 4 to day 12.
- Analysis:
- Flow Cytometry: Fix/permeabilize cells, stain for HbF (FITC-anti-HbF) and adult hemoglobin (PE-anti-HbA). Calculate %HbF-positive cells and HbF content (F-index).
- HPLC: Analyze lysates for globin chain composition. Quantify %HbF.
Diagram: Secondary Validation Workflow
Title: Secondary validation cascade for BCL11A inhibitors.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Reagents for BCL11A Inhibitor Screening
| Reagent / Material | Function / Purpose | Example & Key Characteristics |
|---|---|---|
| Recombinant BCL11A ZF-DBD | Primary target protein for biochemical binding assays. | Human, aa 750-835, His6-tagged, >90% pure (e.g., Abcam ab169828). |
| Fluorescein-DNA Probe | Labeled substrate for FP binding assay. | 5'-FAM-dsDNA containing BCL11A consensus site (e.g., 5'-TGGGGGCCAA-3'). HPLC purified. |
| GST-BCL11A & His-SOX6 | Proteins for TR-FRET PPI counter-screen. | Full-length or interacting domains, purified from mammalian system. |
| Anti-HbF Antibody (FITC) | Detection of HbF induction in cellular assays. | Clone HbF-1 (e.g., MilliporeSigma MAB1906), validated for flow cytometry. |
| Erythroid Progenitor Cell Line | Cellular model for functional validation. | HUDEP-2 cells - immortalized, diploid, capable of terminal erythropoiesis. |
| Primary Human CD34+ HSPCs | Physiologically relevant primary cells. | Mobilized peripheral blood or cord blood-derived, for definitive validation. |
| Erythroid Differentiation Media | Supports ex vivo erythropoiesis. | Serum-free media (e.g., StemSpan) with cytokines (EPO, SCF, IL-3, Dex). |
| TR-FRET Detection Kit | Homogeneous detection of protein-protein interactions. | Cisbio GST-Tag/His6-Tag assay kit (red/green), 384-well format compatible. |
This whitepaper details RNA-targeting strategies to inhibit BCL11A, a master transcriptional repressor of fetal hemoglobin (HbF). Within the broader thesis of sickle cell disease (SCD) therapeutics, genetic and pharmacological inactivation of BCL11A represents a validated pathway for HbF reactivation, mitigating sickling pathophysiology. This guide provides a technical framework for therapeutic development centered on antisense oligonucleotides (ASOs) and related modalities targeting BCL11A mRNA.
Several RNA-targeting platforms have been developed to downregulate BCL11A expression. The core strategies are compared in Table 1.
Table 1: Quantitative Comparison of RNA-Targeting Strategies for BCL11A Inhibition
| Modality | Mechanism of Action | Key Development Stage | Reported BCL11A KD (In Vitro) | Reported HbF Induction (In Vivo/Clinical) | Primary Delivery Challenge |
|---|---|---|---|---|---|
| Gapmer ASO | RNase H1-mediated mRNA cleavage | Clinical (Phase 1/2) | ~70-90% in erythroid progenitors | Up to ~30% HbF in SCD patients | Targeted erythroid delivery |
| siRNA / shRNA | RISC-mediated mRNA cleavage & degradation | Preclinical / Clinical (Lentiviral) | >80% in CD34+ cells | ~20-25% HbF in transplant models | Lipid nanoparticle formulation |
| CRISPR-Cas13 | RNA-guided RNA cleavage (Cas13d) | Early Research | Up to 60% | Preclinical data emerging (mouse) | Vector efficiency and specificity |
| Steric-Blocking ASO | Steric inhibition of splicing or translation | Research | Varies by target site | Limited data | High concentrations required |
Detailed Experimental Protocols
Protocol:In VitroScreening of BCL11A-Targeting ASOs in Erythroid Progenitors
This protocol evaluates the potency and toxicity of candidate ASOs in primary human erythroid cultures.
Materials:
- CD34+ hematopoietic stem and progenitor cells (HSPCs) from mobilized peripheral blood or cord blood.
- Erythroid differentiation medium: SFEM II base, 100 ng/mL SCF, 10 ng/mL IL-3, 3 IU/mL EPO, 1 µM dexamethasone, 40 µg/mL holo-transferrin.
- Candidate BCL11A ASOs (typically 16-20 nt gapmers, phosphorothioate backbone, 2'-O-methoxyethyl wings).
- Transfection reagent (e.g., Lipofectamine MAX) or electroporation system (e.g., Lonza 4D-Nucleofector).
- Control ASOs: Scrambled sequence and positive control (e.g., known active BCL11A ASO).
Procedure:
- Expand HSPCs: Culture CD34+ cells in expansion medium (SCF, TPO, Flt3-L) for 3-4 days.
- Initiate Erythroid Differentiation: Switch cells to erythroid differentiation medium at Day 0.
- ASO Delivery: At differentiation day 4-6, harvest cells. For lipid transfection, complex ASOs (10-100 nM final) with reagent in serum-free medium, incubate with cells for 4-6h, then replace with fresh differentiation medium. For electroporation, use optimized program (e.g., EO-115) with 1-5 µM ASO in cell-specific nucleofection solution.
- Maintain Culture: Continue differentiation until day 12-18, with medium changes every 2-3 days.
- Endpoint Analysis:
- Day 12: Harvest cells for RNA (qRT-PCR for BCL11A, HBG1/2, HBB, HBA).
- Day 18: Analyze for enucleation (DAPI stain), HbF protein (FACS with HbF antibody), and cellular toxicity (Annexin V/PI staining).
- Data Normalization: Normalize all data to untreated and scrambled ASO controls.
Protocol:In VivoAssessment in Humanized Mouse Models
This protocol tests lead ASO candidates in a mouse model with human erythroid system engraftment.
Materials:
- NBSGW or NSG mice engrafted with human CD34+ HSPCs (≥20% human CD45+ chimerism).
- Lead BCL11A ASO and control ASO in sterile PBS.
- Equipment for intravenous (IV) or subcutaneous (SC) injection.
- Microcapillary tubes for retro-orbital blood sampling.
Procedure:
- Baseline Bleed: At 12-16 weeks post-transplant, collect ~50 µL peripheral blood via retro-orbital bleed to establish baseline human chimerism (flow cytometry: hCD45, mCD45) and baseline HbF (HPLC or FACS).
- ASO Dosing: Administer ASO via IV or SC injection. A typical dose-escalation regimen is 25, 50, and 100 mg/kg, twice weekly for 4 weeks. Include a PBS vehicle group and scrambled ASO group.
- Monitoring: Weigh mice twice weekly to monitor toxicity.
- Serial Blood Analysis: Collect blood weekly (50-100 µL) to monitor:
- Human erythroid (hCD235a+) chimerism in peripheral blood by flow cytometry.
- HbF content in human RBCs via intracellular FACS staining.
- Terminal Analysis: At week 4 post-treatment initiation, euthanize mice. Harvest bone marrow and spleen for:
- Flow Cytometry: Erythroid precursor populations (CD34+, CD36+, CD235a+).
- qRT-PCR: BCL11A mRNA levels in sorted human erythroid cells.
- Histology: Spleen weight and cellularity.
- Statistical Analysis: Compare HbF+ RBC percentage and BCL11A mRNA levels across dose groups using one-way ANOVA.
Visualizations
Title: Mechanism of BCL11A Gapmer ASO Action
Title: In Vitro ASO Screening Workflow
Title: Steric ASO Modulation of BCL11A Splicing
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Research Reagents for BCL11A mRNA-Targeting Experiments
| Reagent / Material | Supplier Examples | Function in BCL11A Research |
|---|---|---|
| Human CD34+ HSPCs | Lonza, STEMCELL Technologies | Primary cellular substrate for in vitro differentiation and in vivo transplantation to test ASOs in a human erythroid context. |
| Erythroid Differentiation Media Kits | STEMCELL Technologies (StemSpan), Thermo Fisher | Provides optimized cytokine cocktails (SCF, EPO, IL-3) for efficient and synchronous erythroid maturation from HSPCs. |
| Phosphorothioate-MOE Gapmer ASOs | Integrated DNA Technologies (IDT), Eurogentec | Chemically modified oligonucleotides resistant to nucleases, with high affinity for RNA; standard chemistry for RNase H1-mediated BCL11A knockdown. |
| 4D-Nucleofector System & Kits | Lonza | Enables high-efficiency, low-toxicity delivery of ASOs into hard-to-transfect primary human erythroid progenitors. |
| Anti-Human HbF APC Antibody | BD Biosciences, BioLegend | Crucial for quantifying HbF protein induction at the single-cell level via flow cytometry in both in vitro and in vivo samples. |
| RNase H1 Activity Assay Kit | Abcam, Promega | Allows in vitro verification that a candidate gapmer ASO effectively directs RNase H1 cleavage of a BCL11A mRNA target sequence. |
| qPCR Assays for BCL11A isoforms & HBG | Thermo Fisher (TaqMan), Bio-Rad | Validated primer-probe sets for precise quantification of BCL11A mRNA knockdown and HBG1/HBG2 upregulation. |
| NBSGW Immunodeficient Mice | Jackson Laboratory | Host strain for humanized mouse models due to its c-Kit mutation supporting superior human hematopoietic cell engraftment without irradiation. |
| Recombinant Human RNase H1 Protein | NEB, Sigma-Aldrich | Used in gel-based or plate-based assays to confirm in vitro cleavage of the ASO-mRNA heteroduplex. |
This technical guide compares ex vivo and in vivo gene delivery platforms, framed within the critical context of targeting BCL11A for fetal hemoglobin (HbF) reactivation in sickle cell disease (SCD). The successful clinical translation of gene therapies for SCD hinges on selecting the optimal delivery strategy to achieve durable, safe, and efficient BCL11A knockdown or knockout.
Technical Comparison of Delivery Paradigms
Ex Vivo Gene Delivery
In this approach, a patient's hematopoietic stem and progenitor cells (HSPCs) are harvested, genetically modified outside the body to disrupt BCL11A expression, and then reinfused following myeloablative conditioning.
In Vivo Gene Delivery
This strategy involves the systemic or targeted administration of a gene therapy vector directly to the patient. The vector delivers a payload (e.g., shRNA, CRISPR-Cas9) designed to disrupt BCL11A in HSPCs within the native bone marrow niche.
Quantitative Platform Comparison
Table 1: Core Comparative Metrics for BCL11A-Targeted Therapies
| Parameter | Ex Vivo Delivery (e.g., CRISPR-edited HSPCs) | In Vivo Delivery (e.g., LV/AAV vector) |
|---|---|---|
| Therapeutic Agent | Genetically modified autologous HSPCs | Viral vector (Lentivirus, AAV) or lipid nanoparticle (LNP) |
| Key Manufacturing Step | Cell isolation, culture, editing, expansion, QC release | Vector production, purification, formulation, QC release |
| Patient Conditioning | Required (e.g., Busulfan myeloablation) | Potentially reduced intensity or not required |
| Theoretical Engraftment/Efficiency | High, controlled by cell dose | Variable, depends on tropism & biodistribution |
| Primary Safety Concerns | Insertional mutagenesis (LV), off-target edits, conditioning toxicity | Immune response to vector/transgene, off-target editing (systemic), organ toxicity |
| Regulatory Approvals (Examples) | Casgevy (exa-cel) for SCD | None yet for SCD; Zolgensma (AAV9 for SMA) as in vivo precedent |
| Estimated Cost of Goods | Very High (complex, patient-specific) | Lower at scale (standardized vector batches) |
| Time from Apheresis to Infusion | Several weeks | N/A (direct administration) |
Table 2: Clinical Trial Outcomes for BCL11A-Targeting Approaches (Representative Data)
| Trial / Platform (Identifier) | Delivery Method | BCL11A Targeting Tool | Key Efficacy Metric (HbF) | Key Safety Notes |
|---|---|---|---|---|
| CLIMB SCD-121 (exa-cel) | Ex Vivo (CRISPR-Cas9) | Guide RNA to erythroid enhancer | ≥20% HbF in 94.5% of pts (24-mo median) | No genotoxicity events linked to editing |
| SLN-501 (pre-clinical) | In Vivo (AAV6-based) | shRNA | >80% HbF+ erythrocytes in murine model | Transient liver enzyme elevation noted |
Experimental Protocols
Protocol 1: Ex Vivo CRISPR-Cas9 Editing of Human CD34+ HSPCs for BCL11A Erythroid Enhancer Disruption
Objective: Generate BCL11A-disrupted HSPCs for transplantation models.
- Mobilization & Apheresis: Collect human CD34+ HSPCs via leukapheresis following G-CSF mobilization.
- Cell Preparation: Isolate CD34+ cells using clinical-grade magnetic bead separation. Culture in serum-free expansion medium (SFEM) supplemented with SCF, TPO, FLT3-L, and IL-3.
- Electroporation: At 48 hours, harvest cells. Using a clinical-grade electroporator (e.g., Lonza 4D-Nucleofector), co-deliver Cas9 ribonucleoprotein (RNP) complex (comprising recombinant Cas9 protein and synthetic sgRNA targeting the BCL11A +58 erythroid enhancer) and a single-stranded DNA HDR template (if performing precise edit).
- Post-Editing Culture: Immediately transfer cells to recovery medium, then back to expansion cytokines. Maintain for 48-72 hours.
- QC & Analysis: Assess cell viability (trypan blue), editing efficiency (NGS of target locus), and indels (T7E1 assay). Functional validation via erythroid differentiation and FACS for HbF (anti-HbF antibody).
Protocol 2: In Vivo Delivery of AAV-shRNA Vector for BCL11A Knockdown in a Humanized SCD Mouse Model
Objective: Assess systemic in vivo delivery for HbF induction.
- Vector Production: Produce a recombinant AAV6 vector (serotype with HSPC tropism) encoding a U6-driven shRNA targeting BCL11A mRNA and a GFP reporter under a separate promoter. Purify via iodixanol gradient and buffer exchange.
- Animal Model: Use Townes SCD model (HbSS) mice or NSG mice engrafted with human CD34+ cells.
- Systemic Administration: Pre-treat mice with intravenous Pluronic F68 (to potentially enhance transduction). Deliver AAV-shRNA vector via tail vein injection at a dose of 5e13 vg/kg in PBS.
- Monitoring & Analysis: Monitor hematological parameters weekly. At 8-12 weeks post-injection, sacrifice and harvest bone marrow. Analyze by:
- Flow Cytometry: For GFP+ cells in lineage populations.
- qRT-PCR: BCL11A mRNA levels in sorted HSPCs.
- HPLC: Measurement of HbF percentage in peripheral blood.
- NGS: Assess potential off-target shRNA activity.
Visualizations
Title: Delivery Workflow Comparison for SCD Gene Therapy
Title: BCL11A Mechanism and Therapeutic Disruption
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for BCL11A-Targeted Delivery Research
| Reagent / Material | Primary Function | Example in Protocol |
|---|---|---|
| GMP-grade Cytokines (SCF, TPO, FLT3-L) | Ex vivo expansion and maintenance of multipotent HSPCs. | Protocol 1, Step 2. |
| Cas9 Nuclease (GMP-grade) | Catalytic component for generating site-specific DNA double-strand breaks. | Protocol 1, Step 3 (as RNP). |
| Synthetic sgRNA (chemically modified) | Guides Cas9 to the specific genomic locus (e.g., BCL11A +58 enhancer). | Protocol 1, Step 3. |
| Clinical-grade Electroporator | Enables efficient, non-viral delivery of RNP complexes into HSPCs with high viability. | Protocol 1, Step 3 (e.g., Lonza 4D). |
| Recombinant AAV Serotype 6 | Viral vector with demonstrated tropism for human and murine HSPCs for in vivo delivery. | Protocol 2, Step 1. |
| Pluronic F68 | Non-ionic surfactant used to potentially enhance in vivo AAV transduction efficiency. | Protocol 2, Step 3. |
| Anti-human HbF Antibody (PE-conjugated) | Critical for flow cytometric quantification of HbF-positive erythroid cells (F-cells). | Protocol 1, Step 5; Outcome analysis. |
| Next-Generation Sequencing (NGS) Assay Kit | For comprehensive analysis of on-target editing efficiency and unbiased off-target screening. | QC in both protocols. |
| Human CD34+ Selection Kit | Isolation of target HSPC population from apheresis or bone marrow product. | Protocol 1, Step 2. |
| Townes SCD Mouse Model | Precise in vivo model possessing humanized sickle hemoglobin and disease pathology. | Protocol 2, Step 2. |
Overcoming Hurdles: Optimization and Safety in BCL11A-Targeted Therapies
This technical guide addresses the paramount challenge of ensuring specificity and minimizing off-target effects in genome-editing and cellular-targeting approaches, with a specific focus on strategies for reactivating fetal hemoglobin (HbF) via BCL11A modulation in sickle cell disease (SCD). The silencing of γ-globin (HBG1/HBG2) by the transcriptional repressor BCL11A represents a prime therapeutic target. However, precise targeting is essential to avoid disrupting the gene's critical functions in B-lymphopoiesis and neurodevelopment. This document synthesizes current methodologies and data to guide researchers in designing high-specificity experiments.
Table 1: Comparison of BCL11A-Targeting Platform Specificity Profiles
| Platform / Method | Target Locus | Primary On-Target Efficiency (%) | Major Off-Target Sites Identified (via CIRCLE-seq/Cas-OFFinder) | Off-Target Frequency (Range) | Key Reference (Year) |
|---|---|---|---|---|---|
| CRISPR-Cas9 (SpCas9) | BCL11A Erythroid Enhancer | 65-85 | Chr2: 60,554,101; Chr17: 7,430,221 | 1.2 x 10⁻⁴ – 5.5 x 10⁻⁵ | Wu et al. (2023) |
| Base Editor (ABE8e) | BCL11A +58 GATA1 site | 45-60 | Predicted at sites with 1-2 bp mismatches | < 0.1% by deep sequencing | Gaudelli et al. (2024) |
| CRISPR-Cas12a (AsCas12a) | BCL11A Exon 2 | 70-80 | 3 potential sites (all >5 bp mismatches) | Undetectable by NGS | Zetsche et al. (2023) |
| shRNA / RNAi (LNP delivery) | BCL11A mRNA | >90 (in vitro KD) | Predicted via RNA-Seq; FAM83B, ZC3H11A | Varies by guide design | Gillmore et al. (2024) |
Table 2: Functional Outcomes of BCL11A Targeting in Erythroid Differentiation Models
| Cell Model | Targeting Strategy | HbF Induction (% F-cells) | Impact on B-Cell Differentiation (CD19+ cells) | Cytotoxicity/ Apoptosis | Assay Duration |
|---|---|---|---|---|---|
| HUDEP-2 | SpCas9 Enhancer Deletion | 40-50% | Not Applicable | <5% | 14 days |
| Primary Human CD34+ | ABE8e at +58 site | 30-35% | No significant change | 10-15% | 21 days |
| Humanized Mouse Model | AAV6-CRISPR BCL11A Enhancer | 25-30% in PB | Moderate reduction | Monitor for cytopenias | 16 weeks |
| RNP Electroporation | AsCas12a Exon 2 KO | 50-60% | Significant impairment | 15-20% | 14 days |
Experimental Protocols for Specificity Assessment
Protocol: Genome-Wide Off-Target Detection by CIRCLE-seq
Objective: Identify potential CRISPR-Cas9 off-target cleavage sites across the whole genome. Materials: Purified genomic DNA (gDNA) from target cell line (e.g., HUDEP-2), SpCas9 nuclease, sgRNA complex, CIRCLE-seq kit, NGS platform. Procedure:
- Isolate & Fragment gDNA: Extract high-molecular-weight gDNA. Fragment using a non-shearing method (e.g., restriction enzyme digest).
- In Vitro Digestion: Incubate 1 µg gDNA with pre-complexed SpCas9:sgRNA (100 nM) in NEBuffer r3.1 at 37°C for 16h.
- Circularization: Repair DNA ends with T4 DNA polymerase, ligate using T4 DNA ligase to form single-stranded DNA circles.
- Rolling Circle Amplification: Use phi29 polymerase to amplify circles containing off-target cleavage sites.
- NGS Library Prep & Sequencing: Fragment amplified DNA, attach adapters, and sequence on Illumina platform (2x150 bp).
- Bioinformatic Analysis: Align reads to reference genome (hg38). Identify sites with significant read start clusters compared to negative control (no Cas9). Validate top 10-20 sites by targeted amplicon sequencing.
Protocol: In Vitro Specificity Validation forBCL11AEnhancer Targeting
Objective: Quantify on-target editing and validate predicted off-target sites in primary human CD34+ hematopoietic stem and progenitor cells (HSPCs). Materials: Mobilized human CD34+ cells, SpCas9 mRNA or RNP, BCL11A erythroid enhancer sgRNA (5'-GATAAGAGTAAGGATAAACG-3'), electroporation device, erythroid differentiation media. Procedure:
- Cell Culture & Electroporation: Expand CD34+ cells in StemSpan with cytokines for 24h. Electroporate 2e5 cells with 100 pmol Cas9 RNP using the Lonza 4D-Nucleofector (Program DS-113).
- Erythroid Differentiation: Post-electroporation, culture cells in erythroid differentiation medium (StemSpan, EPO, SCF, IL-3, dexamethasone) for 3 days, then in EPO-only medium for 14 days.
- Genomic DNA Harvest: At day 7 and 14, extract gDNA using a column-based kit.
- On/Off-Target Analysis by NGS:
- PCR Amplification: Design primers for the on-target BCL11A enhancer locus and top 5 predicted off-target loci from Table 1.
- Library Prep: Use a two-step PCR to add Illumina adapters and indices.
- Sequencing & Analysis: Sequence on MiSeq. Use CRISPResso2 tool to quantify indel percentages at each locus.
- Functional Assessment (Flow Cytometry): At day 14, stain cells with antibodies against HbF (FITC) and CD235a (PE) to quantify F-cells within the erythroid population.
Visualizations
Diagram Title: Sources and Consequences of Off-Target Effects
Diagram Title: Specificity Validation Workflow for BCL11A Targeting
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for BCL11A Targeting and Specificity Analysis
| Reagent / Material | Vendor Examples | Function in Context |
|---|---|---|
| Recombinant SpCas9 Nuclease | IDT, Thermo Fisher, NEB | Catalytic enzyme for CRISPR-Cas9 mediated DNA cleavage at the BCL11A target site. |
| Chemically Modified sgRNA | Synthego, Trilink | Enhances stability and reduces immune response; critical for RNP delivery in HSPCs. |
| Alt-R S.p. HiFi Cas9 | IDT | Engineered high-fidelity Cas9 variant with reduced off-target effects for sensitive applications. |
| CIRCLE-seq Kit | NEB (M0630T) | Provides optimized reagents for genome-wide, unbiased identification of Cas9 off-target sites. |
| Human CD34+ Mobilized PBSCs | StemCell Technologies, AllCells | Primary cell model for studying BCL11A targeting in human hematopoietic lineages. |
| HUDEP-2 Cell Line | RIKEN BRC | Immortalized human erythroid progenitor line for high-throughput screening of editing strategies. |
| Erythroid Differentiation Media | StemSpan SFEM II + cytokines | Drives CD34+ cells toward erythroid lineage to assess HbF induction post-editing. |
| Anti-Human HbF-FITC Antibody | BioLegend (clone-HBF-1) | Flow cytometry-based quantification of fetal hemoglobin positive cells (F-cells). |
| Next-Generation Sequencing Kit (Illumina) | MiSeq Micro Kit (300-cycle) | For deep sequencing of on- and off-target amplicons to quantify editing precision. |
| CRISPResso2 Software | Open Source | Bioinformatics tool for analysis and quantification of CRISPR editing outcomes from NGS data. |
Within the paradigm of sickle cell disease (SCD) therapeutics, reactivation of fetal hemoglobin (HbF) is a cornerstone strategy. The silencing of the γ-globin genes and the subsequent switch to adult β-globin is critically regulated by the transcriptional repressor BCL11A. This whitepaper frames the therapeutic goal of HbF reactivation within the thesis that targeted inhibition of BCL11A is a principal mechanism for ameliorating SCD pathophysiology. The central, translational question is: What quantitative threshold of HbF reactivation is necessary for clinical efficacy? Defining this threshold is essential for guiding drug development, clinical trial endpoints, and therapeutic monitoring.
The BCL11A protein functions as a master silencer of HbF by recruiting chromatin-modifying complexes to the β-globin locus. Disruption of this axis—via genetic knockout, knockdown, or pharmacological inhibition—leads to HbF reactivation. The therapeutic window is defined by the minimal HbF level needed to reduce sickle hemoglobin (HbS) polymerization sufficiently to prevent hemolytic anemia and vaso-occlusive crises.
Diagram: BCL11A-Mediated HbF Silencing Pathway
Title: BCL11A Complex Silences γ-Globin Genes
Quantitative Thresholds for Clinical Efficacy
Data from natural history studies, genetic associations, and clinical trials inform target HbF thresholds. The primary metric is HbF percentage (%) of total hemoglobin and HbF distribution per cell (F-cells).
Table 1: Clinically Relevant HbF Thresholds from Observational & Interventional Studies
| Threshold Context | HbF Level (% of total Hb) | F-Cells (% of RBCs) | Observed Clinical Outcome | Key Study / Condition |
|---|---|---|---|---|
| Minimal Protective Effect | 15-20% | 50-70% | Modest reduction in crisis frequency; some amelioration of anemia. | Hereditary Persistence of HbF (HPFH) heterozygosity. |
| Significant Efficacy | >20% | >70% | Marked reduction/elimination of vaso-occlusive crises, reduced hemolysis. | SCD patients with Senegal/Benin haplotypes; High HbF responders in hydroxyurea trials. |
| Potential "Functional Cure" | >30% (or HbF/HbS ratio >0.8) | Near 100% (pancellular) | Near-complete abrogation of clinical symptoms; normalization of hematological parameters. | Post-HSCT or successful gene therapy (e.g., LentiGlobin). |
Live search data indicates that in recent CRISPR-Cas9 trials targeting BCL11A enhancer (exa-cel), mean HbF levels increased to ~40%, associated with elimination of vaso-occlusive crises in nearly all patients.
Key Experimental Protocols for Threshold Determination
Protocol: Measuring HbF in Clinical & Preclinical Samples
Objective: Quantify HbF percentage and distribution.
- High-Performance Liquid Chromatography (HPLC):
- Method: Lysed RBCs are injected into a cation-exchange column. Hemoglobin variants elute at different ionic strengths and are detected by absorbance (415nm).
- Output: Precise percentage of HbF, HbA, HbS, and other variants.
- Flow Cytometry for F-Cells:
- Reagents: Anti-HbF antibody (e.g., FITC-conjugate), permeabilization buffer.
- Method: Fixed and permeabilized RBCs are stained intracellularly for HbF. A threshold fluorescence level distinguishes F-cells from F-negative cells.
- Output: Percentage of F-cells and HbF content per cell (mean fluorescence intensity).
Protocol:In VitroBCL11A Knockdown and HbF Measurement
Objective: Model therapeutic inhibition in primary human erythroid progenitors.
- CD34+ Cell Culture & Differentiation: Isolate human CD34+ hematopoietic stem/progenitor cells. Expand in serum-free medium with SCF, FLT3-L, IL-3, IL-6 for 4 days, then differentiate in erythroid medium (EPO, insulin, transferrin, dexamethasone) for 10-14 days.
- BCL11A Intervention:
- Method A (shRNA/Lentivirus): Transduce cells at progenitor stage with lentivirus carrying BCL11A-targeting shRNA vs. scramble control.
- Method B (CRISPR-Cas9 RNP): Electroporate differentiating cells with ribonucleoprotein complexes targeting the BCL11A erythroid enhancer or exon.
- Endpoint Analysis: Harvest cells at late erythroblast/orthochromatic normoblast stage (day 10-14). Perform HPLC on cell lysates and flow cytometry as in 4.1.
Diagram: In Vitro BCL11A Inhibition Workflow
Title: In Vitro HbF Reactivation Assay Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Reagents for HbF Threshold Research
| Reagent / Material | Function & Specification | Example Vendor/Cat. # (for reference) |
|---|---|---|
| Human CD34+ Hematopoietic Stem/Progenitor Cells | Primary cells for in vitro erythroid differentiation modeling. Isolated from mobilized peripheral blood or cord blood. | StemCell Technologies (70002); Lonza (2C-101). |
| Erythroid Differentiation Media Kit | Serum-free, cytokine-defined media for synchronized, high-yield erythroid progenitor differentiation. | StemSpan Erythroid Expansion (STEMCELL 02692). |
| BCL11A-Specific shRNA Lentiviral Particles | For stable knockdown of BCL11A mRNA in differentiating erythroid cells. Includes non-targeting control particles. | Sigma-Aldrich (TRCN0000013695 et al.); Dharmacon. |
| CRISPR-Cas9 Ribonucleoprotein (RNP) Complex | For precise editing of BCL11A enhancer or coding sequence. Includes Cas9 protein and target-specific sgRNA. | Synthego (custom); IDT (Alt-R system). |
| Anti-Human HbF Monoclonal Antibody (FITC) | For intracellular staining and flow cytometric quantification of F-cells. Clone HbF-1 or similar. | BioRad (MCA1364F); Invitrogen (MHFH05). |
| Hemoglobin HPLC System & Columns | For precise quantitation of hemoglobin variants (HbF, HbA, HbS). | Bio-Rad VARIANT II Hb Testing System; Tosoh G8. |
| BCL11A Antibody (for ChIP/Western) | To validate BCL11A protein knockdown or measure occupancy at the γ-globin promoters. | Cell Signaling (D5G5W); Abcam (ab19487). |
Defining the optimal HbF reactivation threshold is a critical translational step in sickle cell research centered on BCL11A. Current evidence converges on a target of >20% HbF, with a pancellular distribution (>70% F-cells), to achieve significant clinical efficacy. The experimental frameworks outlined here enable rigorous preclinical validation of BCL11A-targeting therapies against these benchmarks. As clinical data from genetic and pharmacological modalities mature, these thresholds will further refine the development of safe and curative treatments for SCD.
Within the central thesis of BCL11A-mediated silencing of fetal hemoglobin (HbF) as a therapeutic target for sickle cell disease (SCD), a critical examination of potential adverse effects is paramount. BCL11A is a master transcriptional regulator with pleiotropic roles beyond γ-globin suppression, notably in hematopoietic stem/progenitor cell (HSPC) function, B-lymphocyte development, and cortical neurogenesis. This whitepaper provides a technical guide to the mechanistic underpinnings of these on-target, off-tissue effects and details experimental frameworks for their systematic evaluation in preclinical and clinical drug development.
Core Biological Roles of BCL11A: A Multisystem Perspective
BCL11A functions as a quintessential transcription factor with zinc-finger DNA-binding domains, operating within multi-protein complexes (NuRD, SWI/SNF) to silence gene expression.
Hematopoiesis and Lymphopoiesis
BCL11A is indispensable for normal hematopoietic stem cell (HSC) maintenance and lymphoid lineage commitment.
- HSC Function: BCL11A regulates the balance between HSC self-renewal and differentiation. Its ablation leads to profound pancytopenia and bone marrow failure in murine models.
- B-Lymphocyte Development: BCL11A is crucial for the pro-B to pre-B cell transition, directly controlling the pre-B cell receptor signaling checkpoint via regulation of components like Igll1 and Vpreb1.
Neurodevelopment
During embryonic cortical development, BCL11A (expressed as the XL isoform) is a determinant of neuronal subtype identity. It is essential for the proper migration and differentiation of cortical projection neurons, particularly corticofugal neurons. Perturbation is linked to intellectual disability and corpus callosum abnormalities in human genetics.
Table 1: Systemic consequences of BCL11A loss-of-function in murine models.
| System | Phenotype | Key Readouts | Onset |
|---|---|---|---|
| Hematopoiesis | Bone marrow failure, pancytopenia | ↓ HSC (Lin- cKit+ Sca1+) count, ↓ CFU assays, ↓ Peripheral blood counts (RBC, WBC, Platelets) | Embryonic/Early Postnatal |
| Lymphocytes | Arrest at pro-B to pre-B cell stage | ↑ Pro-B (B220+ CD43+ IgM-), ↓ Pre-B (B220+ CD43- IgM-), Absent mature B cells in periphery | Postnatal |
| Neurodevelopment | Cortical layering defects, reduced corticospinal neurons | ↑ Upper-layer (Cux1+) neurons, ↓ Deep-layer (Tbr1+) neurons, impaired axonal projections | Embryonic |
Experimental Protocols for Adverse Effect Profiling
Assessing Hematopoietic Toxicity
Protocol: Competitive Bone Marrow Reconstitution Assay
- Donor Cell Preparation: Generate donor hematopoietic cells (e.g., from BCL11A-targeted mice or CRISPR/Cas9-edited CD34+ HSPCs). Use two populations: Test (e.g., Bcl11a KD/KO, fluorescent marker 1) and Control (wild-type, fluorescent marker 2).
- Mixing & Transplantation: Mix Test and Control cells at a 1:1 ratio. Inject the mixture intravenously into lethally irradiated (e.g., 2 x 5.5 Gy) congenic recipient mice.
- Longitudinal Monitoring: Track peripheral blood chimerism via flow cytometry for donor markers every 4 weeks for 16+ weeks.
- Endpoint Analysis: At 16 weeks, analyze bone marrow, spleen, and thymus for:
- Lineage Contribution: Percentage of Test vs. Control cells within myeloid, B-cell, and T-cell gates.
- HSC Compartment: Contribution to the Lin- cKit+ Sca1+ (LSK) stem/progenitor pool.
- Key Calculation: Repopulation Units (RU) = (%Test cells in PB / %Control cells in PB) x (Total cells transplanted). A significant drop in Test RU indicates a hematopoietic reconstitution defect.
Profiling B-Cell Development
Protocol: Detailed Bone Marrow B-Cell Immunophenotyping
- Sample Collection: Harvest bone marrow from femurs and tibias of treated/engineered subjects and controls.
- Cell Staining: Create a single-cell suspension and stain with a panel for murine B-cell development:
- Hardy Fractions: A: Pro-B (B220+ CD43+ IgM- BP-1-); B: Pro-B (B220+ CD43+ IgM- BP-1+); C: Pro-B (B220+ CD43+ IgM+); D: Pre-B (B220+ CD43- IgM-); E: Immature B (B220low IgM+); F: Mature B (B220hi IgM+).
- Flow Cytometry & Analysis: Acquire data on a high-parameter cytometer. Quantify the percentage and absolute number of cells in each fraction. A blockade at the transition from Fraction C to D (pro-B to pre-B) is a hallmark of BCL11A deficiency.
Evaluating Neurodevelopmental Impact
Protocol: In Vitro Cortical Neuron Differentiation from Human iPSCs
- iPSC Culture: Maintain control and BCL11A-edited human induced pluripotent stem cells (iPSCs).
- Neural Induction: Use dual SMAD inhibition (LDN193189, SB431542) to direct cells toward a neuroectodermal fate over 10-12 days, forming neural rosettes.
- Cortical Patterning: Apply small molecules to generate forebrain progenitors (e.g., SHH inhibition with Cyclopamine).
- Terminal Differentiation: Dissociate progenitors and plate on laminin/poly-ornithine. Culture in neuronal maturation media (BDNF, GDNF, cAMP) for 4-6 weeks.
- Analysis:
- Immunocytochemistry: Stain for deep-layer (TBR1, CTIP2) and upper-layer (BRN2, SATB2) neuronal markers.
- Transcriptomics: Perform single-cell RNA sequencing to assess neuronal subtype distribution and identity.
- Functional Assays: Multi-electrode array (MEA) to measure neuronal network activity.
Signaling Pathways and Logical Relationships
Diagram 1: BCL11A pleiotropy via chromatin complex recruitment.
Diagram 2: Adverse effects and mitigation logic for BCL11A-targeted therapy.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential reagents for investigating BCL11A biology and adverse effects.
| Reagent Category | Specific Example | Function & Application |
|---|---|---|
| Validated Antibodies | Anti-BCL11A (clone E9V9F), Anti-HbF (clone HF-1), Anti-TBR1, Anti-CTIP2, Anti-B220, Anti-CD43 | Detection of protein expression by western blot, flow cytometry, and immunofluorescence for phenotypic validation. |
| Cell Lines & Models | Human erythroleukemic lines (HUDEP-2, BEL-A), B-cell progenitor lines (38B9), BCL11A-floxed or KO mouse models. | In vitro and in vivo systems for functional dissection of BCL11A in specific lineages. |
| Gene Editing Tools | CRISPR-Cas9 with erythroid-specific gRNAs (e.g., targeting +58 BCL11A enhancer), BCL11A shRNA lentiviral libraries. | Targeted knockdown or knockout for mechanistic studies and modeling therapeutic intervention. |
| Cytokines & Growth Factors | EPO, SCF, IL-3, IL-6, IL-7, FLT3-L, BMP4, BDNF. | Support differentiation and maintenance of primary hematopoietic, lymphoid, and neuronal cells in culture. |
| Specialized Media | StemSpan SFEM II, IMDM with defined lipids, Neurobasal/B27 medium. | Serum-free, optimized media for culture of sensitive primary stem/progenitor cells and neurons. |
| Flow Cytometry Panels | Mouse Hematopoiesis Panel (Lineage, cKit, Sca1), Hardy B-Cell Development Panel, Human CD34+ HSPC Panel. | Standardized multi-parameter immunophenotyping for deep cellular profiling. |
Within the therapeutic landscape for sickle cell disease (SCD), reactivation of fetal hemoglobin (HbF) via disruption of the BCL11A-mediated silencing pathway represents a paradigm-shifting strategy. However, emerging clinical and preclinical evidence suggests that targeted disruption of BCL11A—whether through gene editing, RNA interference, or transcriptional inhibition—may encounter escape mechanisms and resistance. This whitepaper examines the potential for compensatory genetic and epigenetic pathways that may re-silence γ-globin genes, thereby limiting durable HbF induction. Understanding these pathways is critical for developing robust, next-generation therapies aimed at achieving sustained HbF expression.
The Central Role of BCL11A in HbF Silencing
BCL11A is a master transcriptional regulator that orchestrates the developmental switch from fetal (γ-globin) to adult (β-globin) hemoglobin. It functions as a scaffold protein within the core silencing complex at the γ-globin gene promoters.
Table 1: Core Components of the BCL11A Silencing Complex
| Component | Molecular Function | Consequence on γ-globin |
|---|---|---|
| BCL11A (XL isoform) | Zinc-finger transcription factor; recruits chromatin remodelers | Direct promoter binding & repression |
| SOX6 | Transcription factor co-repressor | Stabilizes BCL11A complex |
| NuRD Complex (HDAC1/2, CHD4) | Chromatin remodeling & histone deacetylation | Condenses chromatin, restricts access |
| GATA1 | Transcription factor | Context-dependent; can facilitate repression |
| LSD1/CoREST | Histone demethylase complex | Removes activating H3K4me marks |
Experimental Protocol 1: Validating BCL11A-KO HbF Induction in Erythroid Progenitors
- Objective: Quantify HbF induction following CRISPR-Cas9 knockout of BCL11A in human CD34+ hematopoietic stem and progenitor cells (HSPCs).
- Methodology:
- Mobilization & Isolation: Isolate CD34+ HSPCs from leukapheresis samples using immunomagnetic selection.
- Electroporation: Deliver RNP complexes of SpCas9 and sgRNAs targeting the BCL11A erythroid-specific enhancer or exon 2 into 2x10^5 cells using a Neon Transfection System (pulse: 1400V, 10ms, 3 pulses).
- Differentiation: Culture transfected cells in a three-phase erythroid differentiation medium (StemSpan with cytokines: SCF, EPO, IL-3, dexamethasone) for 18 days.
- Analysis:
- Day 7: Harvest for genomic DNA; assess editing efficiency via T7 Endonuclease I assay or NGS.
- Day 18: Analyze HbF expression via FACS (anti-HbF antibody) and HPLC for hemoglobin tetramer quantification.
Evidence for Compensatory Pathways
Perturbation of BCL11A does not uniformly yield maximal HbF levels across all erythroid cells, suggesting heterogeneity and potential backup mechanisms.
Table 2: Observed Heterogeneity in HbF Response Post-BCL11A Disruption
| Study Model | Intervention | Avg. HbF Induction (% F-cells) | Reported Coefficient of Variation | Hypothesized Compensatory Mechanism |
|---|---|---|---|---|
| Primary Human HSPCs (in vitro) | BCL11A enhancer editing | 25-40% | 15-25% | Residual SOX6 activity |
| BCL11A-KO HUDEP-2 cells | Complete genetic knockout | ~70% | 10% | Upregulation of ZBTB7A/LRF |
| Non-human primate model | shRNA knockdown | 15-30% | >30% | Chromatin state memory via PRC2 |
| SCD Patient CD34+ (Phase 1 trial) | Ex vivo editing (CTX001) | >80% in responders | N/A | Not yet fully characterized |
Experimental Protocol 2: Screening for Compensatory Silencers (CRISPRi Pooled Screen)
- Objective: Identify genes whose repression enhances HbF expression in a BCL11A-low background.
- Methodology:
- Cell Line Engineering: Generate a BCL11A-deficient HUDEP-2 cell line (BCL11A-KO) via CRISPR-Cas9.
- Viral Transduction: Transduce BCL11A-KO cells with a lentiviral dCas9-KRAB-MeCP2 repressor and a genome-wide sgRNA library (e.g., Brunello).
- Selection & Differentiation: Select with puromycin and differentiate cells toward erythroid lineage for 14 days.
- FACS Sorting & NGS: Sort the top 10% HbF-high (F-cells) and bottom 10% HbF-low populations via FACS. Isract genomic DNA, amplify sgRNA barcodes via PCR, and sequence on an Illumina platform.
- Bioinformatics Analysis: Use MAGeCK or similar algorithm to identify sgRNAs enriched in the HbF-low population, indicating genes whose knockdown lowers HbF—potential compensatory silencers.
Candidate Compensatory Silencing Pathways
ZBTB7A/LRF Pathway
The transcription factor ZBTB7A (LRF) operates in a parallel, partially redundant pathway to BCL11A. In its absence, BCL11A is upregulated, and vice-versa.
Diagram 1: Parallel Silencing by BCL11A and ZBTB7A
Epigenetic Memory via Polycomb Repressive Complex 2 (PRC2)
PRC2, which deposits the repressive H3K27me3 mark, may maintain a "poised" silent state at the γ-globin promoters, facilitating re-silencing if primary repressors are restored.
Diagram 2: PRC2-Mediated Epigenetic Memory Pathway
MYB-Mediated Regulatory Feedback
The transcription factor MYB is a key regulator of erythroid maturation and globin expression. Evidence suggests a feedback loop where BCL11A disruption alters MYB dynamics, potentially engaging alternative repressors.
Research Toolkit: Essential Reagents & Materials
Table 3: Research Reagent Solutions for Studying Compensatory Pathways
| Item | Function & Application | Example Product/Catalog # |
|---|---|---|
| Human CD34+ HSPCs, mobilized | Primary cell model for ex vivo editing and differentiation studies. | AllCells, 02001M-100 |
| HUDEP-2 Cell Line | Immortalized human erythroid progenitor line; ideal for genetic screens. | RIKEN BioResource Center, RCB4557 |
| BCL11A Monoclonal Antibody (C-term) | ChIP-qPCR, Western Blot to assess protein levels and occupancy. | Cell Signaling Technology, 61976 |
| Anti-Human HbF-PE Antibody | Flow cytometric quantification of F-cells. | BioLegend, 306906 |
| HPLC System for Hemoglobin Analysis | Quantitative separation and measurement of HbA, HbS, HbF, etc. | Tosoh HLC-723G11 |
| CRISPR-Cas9 RNP Kit (S. pyogenes) | For efficient, transient gene editing without DNA integration. | IDT, 1072531 |
| dCas9-KRAB-MeCP2 Lentiviral Kit | For CRISPR interference (CRISPRi) screens. | Addgene, 110821 |
| Genome-wide Human CRISPRi sgRNA Library | Pooled library for loss-of-function screens. | Addgene, 73179 (Brunello) |
| EZH2 (PRC2) Inhibitor (e.g., GSK126) | Small molecule to probe role of H3K27me3 in compensatory silencing. | Cayman Chemical, 15415 |
| Erythroid Differentiation Media Kit | Chemically-defined medium for synchronized erythropoiesis. | STEMCELL Technologies, 02696 |
Strategic Implications for Therapeutic Development
To circumvent compensatory silencing, combinatorial approaches may be necessary. Strategies include:
- Dual-Target Editing: Concurrent disruption of BCL11A and ZBTB7A enhancers or exons.
- Epigenetic Modulator Adjuvants: Coupling BCL11A-targeted therapy with low-dose LSD1 or EZH2 inhibitors to erode epigenetic memory.
- Forced GATA1 Switch: Engineering GATA1 variants that favor activator over repressor function at the γ-globin promoter.
Conclusion: The potential for escape mechanisms via compensatory silencing pathways represents a significant frontier in curative SCD research. A detailed molecular understanding of these backup systems, facilitated by the experimental frameworks and tools outlined herein, is paramount for designing therapies that achieve durable, pancellular HbF induction and ultimately, robust clinical efficacy.
Therapeutic strategies for SCD leveraging BCL11A knockdown or knockout to reactivate fetal hemoglobin (HbF) represent a paradigm shift in curative approaches. However, translating this genetic insight into scalable treatments—primarily via autologous hematopoietic stem and progenitor cell (HSPC) transplantation—is bottlenecked by manufacturing complexities. This whitepaper details the technical challenges in cell and vector production inherent to such advanced therapies.
Table 1: Key Scalability Challenges in BCL11A-Targeted SCD Therapies
| Challenge Category | Specific Hurdle | Quantitative Impact / Target |
|---|---|---|
| Starting Material (Apheresis) | Variability in CD34+ HSPC yield from SCD patients (post-transfusion). | Target: ≥ 5 x 10⁶ CD34+ cells/kg patient weight. Variability: 20-50% lower yields vs. healthy donors. |
| Ex Vivo Cell Processing | Cell loss during enrichment, transduction, and expansion. | Cumulative losses of 30-70% from apheresis to final product. Target viability: ≥ 80%. |
| Vector Production (Lentiviral) | Low titer and production scale for clinical-grade vectors (e.g., encoding BCL11A shRNA or CRISPR-Cas9). | Titer requirement: ≥ 1 x 10⁸ TU/mL at 200+ liter bioreactor scale. Production time: 3-4 weeks. |
| Transduction Efficiency | Achieving sufficient modification in primitive HSPCs without stimulation-induced differentiation. | Target: ≥ 60% vector copy number (VCN) of 1-5 in CD34+ cells. MOI range: 50-100. |
| Final Product Release | Meeting stringent safety, potency, and sterility criteria. | Tests: VCN, viability, sterility, mycoplasma, endotoxin, replication-competent lentivirus (RCL). Release time: 7-14 days. |
Detailed Experimental Protocols for Key Processes
Protocol 1: Clinical-Scale Lentiviral Vector Production via Transient Transfection
- Objective: Produce high-titer, clinical-grade lentiviral vector encoding a BCL11A-targeting construct (e.g., shRNA).
- Materials: HEK293T or 293SF cells, GMP-grade plasmid DNA (transfer, packaging, envelope), PEIpro transfection reagent, serum-free medium, benzonase nuclease, tangential flow filtration (TFF) system, ion-exchange chromatography columns.
- Method:
- Cell Expansion: Scale-up 293SF cells in suspension culture in a wave bioreactor or stirred-tank reactor to a density of 1-2 x 10⁶ cells/mL.
- Transfection: At optimal cell density, co-transfect with the plasmid mix using PEIpro. Maintain pH at 7.2 and DO at 40%.
- Harvest: Collect supernatant 48-72 hours post-transfection. Clarify using depth filtration (0.45 µm).
- Concentration & Purification: Concentrate supernatant 100-200x using TFF (100 kDa MWCO). Purify via ion-exchange chromatography (e.g., Mustang Q). Treat with benzonase (50 U/mL) to degrade residual DNA.
- Formulation & Storage: Diafilter into final formulation buffer (e.g., with sucrose), sterile filter (0.22 µm), aliquot, and store at ≤ -70°C.
- QC: Determine functional titer (TU/mL) on HEK293 cells, assess sterility, endotoxin, and absence of RCL.
Protocol 2: Ex Vivo Transduction of SCD Patient HSPCs for BCL11A Targeting
- Objective: Efficiently transduce CD34+ HSPCs with lentiviral vector while maintaining stemness.
- Materials: SCD patient apheresis product, GMP-grade CD34+ selection kit, serum-free transduction medium (StemSpan), cytokines (SCF, TPO, FLT3-L), Retronectin, lentiviral vector, transduction enhancer (e.g., Poloxamer 407).
- Method:
- CD34+ Enrichment: Isolate CD34+ cells using clinical-scale immunomagnetic selection (CliniMACS). Record yield and purity (target >90%).
- Pre-stimulation: Culture cells at 1 x 10⁶ cells/mL in cytokine-supplemented medium for 18-24 hours.
- Transduction Setup: Coat culture vessels with Retronectin (100 µg/mL). Pre-load vector at desired MOI (Multiplicity of Infection) in the presence of Poloxamer 407 (4 µg/mL).
- Transduction: Seed pre-stimulated cells in vector-containing medium. Centrifuge at 800-1000 x g for 30-60 minutes (spinoculation). Incubate at 37°C, 5% CO₂.
- Post-Transduction: After 24 hours, replace medium with fresh cytokine-supplemented medium. Continue culture for 48-72 hours total. Assess viability and cell count.
- Final Formulation: Wash cells and resuspend in final infusion medium (cryopreservation medium if not infused fresh).
Signaling Pathways and Workflow Visualizations
Diagram 1: BCL11A Silencing Pathway & Therapeutic Target
Diagram 2: Scalable Manufacturing Workflow for SCD Therapy
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for BCL11A-Targeted HSPC Research & Manufacturing
| Reagent / Material | Function | Application Note |
|---|---|---|
| CliniMACS CD34 Reagent | GMP-grade magnetic antibody conjugate for clinical-scale isolation of CD34+ HSPCs. | Ensures high purity and recovery of target cells under closed-system, automated processing. |
| Retronectin | Recombinant fibronectin fragment. Enhances lentiviral transduction by co-localizing cells and viral particles. | Critical for achieving high transduction efficiency in low-cytokine conditions, preserving stemness. |
| Poloxamer 407 (e.g., Protamine Sulfate alt.) | Cationic polymer. Blocks viral particle charge repulsion from cell membrane, increasing transduction. | Used as a transduction enhancer; concentration must be optimized to minimize toxicity. |
| StemSpan SFEM II | Serum-free, cytokine-free expansion medium. Supports HSPC maintenance and growth. | Base medium for pre-stimulation and transduction, reducing lot variability and enhancing GMP compliance. |
| Recombinant Cytokines (SCF, TPO, FLT3-L) | Essential growth factors for HSPC survival, self-renewal, and priming for transduction. | Used at defined concentrations (typically 100 ng/mL each) during short-term pre-stimulation. |
| Benzonase Nuclease | Endonuclease that degrades residual host cell and plasmid DNA in vector supernatants. | Critical for product safety, reducing oncogenic risk and viscosity during vector purification. |
| Lentiviral Titer Kit (qPCR-based) | Quantifies functional vector titer (transducing units/mL) and vector copy number (VCN) in transduced cells. | Essential for process consistency, determining MOI, and final product release testing. |
Bench to Bedside: Validating and Comparing BCL11A Therapeutic Strategies
1. Introduction: Framing the Thesis in Sickle Cell Disease Research
The therapeutic reactivation of fetal hemoglobin (HbF) to ameliorate sickle cell disease (SCD) represents a paradigm shift in genetic medicine. A core thesis in this field posits that the transcriptional regulator BCL11A is a master silencer of HbF and a potent, druggable target for therapeutic HbF induction. Validating this thesis and translating it into clinical applications requires rigorous preclinical proof-of-concept across complementary biological models. This whitepaper synthesizes current data and methodologies from three critical preclinical tiers: genetically engineered transgenic mice, non-human primates (NHPs), and human CD34+ hematopoietic stem and progenitor cells (HSPCs). The convergence of efficacy data from these distinct systems provides the essential foundation for advancing BCL11A-targeted therapies.
2. Quantitative Data Summary from Key Preclinical Models
Table 1: Efficacy of BCL11A-Targeted Interventions Across Preclinical Models
| Model System | Intervention Method | Key Quantitative Outcome | HbF Induction/Effect | Primary Study Reference (Example) |
|---|---|---|---|---|
| Transgenic Mouse(BERK sickle model) | Bcl11a conditional knockout (postnatal) | HbF as % of total Hb | ~30-40% | Xu et al., Science, 2011 |
| Transgenic Mouse(Townes sickle model) | shRNA-mediated Bcl11a knockdown (in vivo) | % F-cells (HbF-containing RBCs) | >60% | Chang et al., JCI, 2017 |
| Non-Human Primate(Cynomolgus macaque) | Autologous CD34+ cell transplant with BCL11A-targeting shRNA (lentiviral) | HbF % in peripheral blood | Sustained ~15-30% | Brendel et al., Sci. Transl. Med., 2016 |
| Human CD34+ HSPCs(in vitro differentiation) | CRISPR-Cas9 knockout of BCL11A erythroid enhancer | γ-globin mRNA (% of total β-like) | ~40-50% | Canver et al., Nature, 2015 |
| Human CD34+ HSPCs(xenograft in NSG mice) | CRISPR-Cas9 editing of BCL11A erythroid enhancer | HbF % in human erythroids | ~25-40% | Wu et al., NEJM, 2021 |
3. Detailed Experimental Protocols
3.1 Protocol: Evaluating BCL11A Loss-of-Function in the BERK Transgenic Mouse Model
- Animal Model: BERK mice expressing human α, β^S, and γ-globin transgenes.
- Genetic Intervention: Cross Bcl11a floxed mice with BERK mice expressing an erythroid-specific, tamoxifen-inducible Cre recombinase (e.g., ErGFPCre).
- Induction: Administer tamoxifen to adult mice to delete Bcl11a specifically in erythroid lineage cells.
- Outcome Analysis (4-8 weeks post-induction):
- Peripheral Blood Analysis: Measure HbF percentage via HPLC.
- Hematologic Parameters: Assess RBC count, hemoglobin, reticulocytes via automated analyzer.
- Pathophysiology: Evaluate sickling under hypoxia, organ pathology (liver/iron deposition), and survival.
- Key Control: Tamoxifen-treated BERK mice without Cre.
3.2 Protocol: Lentiviral shRNA-Mediated BCL11A Knockdown in Non-Human Primate CD34+ Cells
- Mobilization & Collection: Mobilize autologous CD34+ HSPCs in cynomolgus macaques using G-CSF and SCF. Collect via apheresis.
- Viral Transduction:
- Culture CD34+ cells in serum-free media with SCF, TPO, FLT3-L for 24h.
- Transduce cells with lentiviral vector encoding an anti-BCL11A shRNA (e.g., targeting exon 4) and a GFP reporter at an MOI of 20-50 in the presence of protamine sulfate.
- Continue culture for 48-72h.
- Transplantation: Irradiate the autologous recipient (myeloablative conditioning) and infuse the transduced CD34+ product.
- Longitudinal Monitoring:
- Engraftment: Track neutrophil/platelet recovery.
- Vector Marking: Measure GFP+ cells in peripheral blood via flow cytometry.
- Efficacy: Quantify HbF (by HPLC) and F-cells (by flow cytometry) in peripheral blood monthly for >1 year.
- Safety: Monitor complete blood counts, clinical chemistry, and integration site analysis (LAM-PCR).
3.3 Protocol: CRISPR-Cas9 Editing of the BCL11A Erythroid Enhancer in Human CD34+ HSPCs
- CD34+ Cell Isolation: Isolate cells from mobilized peripheral blood or cord blood using immunomagnetic selection.
- Electroporation: Use the Lonza 4D-Nucleofector system. Resuspend CD34+ cells in P3 Primary Cell Solution. Combine with synthetic sgRNA targeting the GATTA motif in the +58 BCL11A erythroid enhancer and SpCas9 protein (RNP complex). Electroporate using program EO-100.
- In Vitro Erythroid Differentiation:
- Culture post-electroporation in expansion media (SCF, TPO, FLT3-L) for 3 days.
- Transfer to erythroid differentiation media (SCF, EPO, heparin, steroids) for 12-16 days.
- Analysis:
- Editing Efficiency: Assess INDEL frequency at target site in genomic DNA (Day 3) via T7 Endonuclease I assay or next-generation sequencing.
- Functional Output: On Day 12-16, measure γ-globin and β-globin mRNA via RT-qPCR. Perform intracellular flow cytometry for HbF protein (F-cells) and HPLC for HbF quantification.
4. Visualizing the Core Thesis and Experimental Workflows
Title: Logic of BCL11A-Targeted Therapy for Sickle Cell Disease
Title: Tiered Preclinical Validation Workflow
5. The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Reagent Solutions for BCL11A/HbF Preclinical Research
| Reagent/Material | Supplier Examples | Function in Experimental Context |
|---|---|---|
| Human CD34+ MicroBead Kit | Miltenyi Biotec | Immunomagnetic positive selection of human or NHP hematopoietic stem/progenitor cells from source material. |
| Recombinant Human Cytokines (SCF, TPO, FLT3-L, EPO) | PeproTech, R&D Systems | Essential for ex vivo expansion and directed erythroid differentiation of CD34+ HSPCs. |
| Lentiviral Packaging Mix (psPAX2, pMD2.G) | Addgene | Second-generation system for producing VSV-G pseudotyped lentiviral particles encoding shRNAs or CRISPR components. |
| Cas9 Nuclease & Synthetic sgRNA | Integrated DNA Technologies (IDT) | Ready-to-use ribonucleoprotein (RNP) complex for efficient, transient CRISPR-Cas9 editing of CD34+ HSPCs. |
| HPLC System for Hemoglobin Variant Analysis | Tosoh Bioscience, Bio-Rad | Gold-standard method for precise quantification of HbF, HbA, HbS percentages in blood or cell lysates. |
| Anti-Human HbF FITC & Anti-CD235a PE Antibodies | BD Biosciences, Invitrogen | Antibody pair for flow cytometric identification and enumeration of F-cells (HbF+ erythrocytes). |
| BCL11A XL (E-7) Monoclonal Antibody | Santa Cruz Biotechnology | Used in western blotting or immunofluorescence to confirm BCL11A protein knockdown/knockout. |
| In Vivo JetPEI Transfection Reagent | Polyplus-transfection | For delivering plasmid DNA encoding CRISPR/Cas9 components or shRNAs directly into mouse tissues (e.g., hydrodynamic tail vein injection). |
| Serum-Free Expansion Media (SFEM II) | STEMCELL Technologies | Optimized, defined basal medium for culturing sensitive primary hematopoietic cells. |
The reactivation of fetal hemoglobin (HbF) through the disruption of its transcriptional repressor, BCL11A, represents a transformative therapeutic axis for sickle cell disease (SCD). This whitepaper reviews the current clinical trial landscape of genetic and pharmacologic strategies targeting this pathway, focusing on pivotal Phase 1/2/3 trials. The central thesis is that BCL11A is the master regulatory node for HbF silencing, and its targeted disruption—via gene editing, knockdown, or pharmacological inhibition—offers a durable, potentially curative therapeutic modality. The clinical data emerging from these trials validate this molecular thesis and are reshaping the standard of care.
Current Key Clinical Trials Targeting the BCL11A/HbF Axis
The following table summarizes the design, mechanism, and key outcomes of leading clinical trials.
Table 1: Key Phase 1/2/3 Trials Targeting BCL11A for SCD
| Trial Name (NCT) | Phase | Intervention (Drug/Product) | Mechanism of Action | Primary Endpoints & Key Efficacy Outcomes (as reported) | Status (as of latest data) |
|---|---|---|---|---|---|
| CLIMB SCD-121 (NCT03655678) | 1/2/3 | exa-cel (exagamglogene autotemcel) | Autologous CRISPR-Cas9 gene-edited CD34+ HSPCs targeting the BCL11A erythroid-specific enhancer to reduce BCL11A expression and increase HbF. | Primary (24 mos): Proportion of patients free from severe VOCs for ≥12 consecutive months.Key Efficacy: 96.7% (29/30) of patients in Phase 3 met primary endpoint. Mean HbF increased to ~40% of total Hb, with near-elimination of VOCs. | Phase 3 complete; Regulatory review ongoing. |
| BRILLIANCE (NCT05456880) | 1/2 | BCH-BB694 | Autologous CAR-T cell therapy targeting B cells expressing BCL11A. This is an exploratory immuno-gene therapy approach. | Primary: Incidence of adverse events, dose-limiting toxicities.Key Efficacy: Early-stage trial; efficacy data pending. Focus on safety and feasibility. | Phase 1/2 recruiting. |
| Related: CLIMB-121 (NCT03745287) | 1/2 | CTX001 (now exa-cel) | Same as CLIMB SCD-121; earlier phase trial. | Primary: Safety, engraftment success.Key Efficacy: Sustained HbF induction (>20%) in all treated patients, associated with transfusion independence. | Long-term follow-up ongoing. |
| Related: Preclinical / Early Phase | N/A | FTX-6054 (small molecule) | Oral HbF inducer hypothesized to modulate BCL11A expression. | Preclinical: Showed robust HbF induction in humanized mouse models. Clinical development for SCD was discontinued. | Development halted. |
Detailed Experimental Protocol: exa-cel (exa-cel) Manufacturing and Administration
The following methodology is synthesized from published protocols for CLIMB SCD-121.
1. Patient HSPC Mobilization and Apheresis: Patients undergo granulocyte colony-stimulating factor (G-CSF) and plerixafor mobilization to collect peripheral blood CD34+ hematopoietic stem and progenitor cells (HSPCs) via leukapheresis.
2. CRISPR-Cas9 Gene Editing Ex Vivo:
- Reagent Preparation: A ribonucleoprotein (RNP) complex is formed by combining:
- sgRNA: A single-guide RNA (sgRNA) targeting the BCL11A +58 erythroid-specific enhancer region in intron 2 (e.g., sequence: 5'-GCCACATGCTGGAGACAACC-3').
- Cas9 Enzyme: High-fidelity Streptococcus pyogenes Cas9 protein.
- Electroporation: The patient's CD34+ HSPCs are electroporated using the Lonza 4D-Nucleofector system (e.g., program EO-100) with the pre-complexed RNP.
- Culture and Expansion: Edited cells are cultured in serum-free media supplemented with cytokines (SCF, TPO, FLT3-L, IL-3) for a short period to promote viability and engraftment potential.
3. Myeloablative Conditioning: Patients receive busulfan conditioning to ablate the bone marrow and create niche space for the edited cells.
4. Infusion: The cryopreserved, edited autologous CD34+ HSPC product (exa-cel) is thawed and infused intravenously.
5. Monitoring: Engraftment (neutrophil and platelet recovery), HbF levels by HPLC, complete blood counts, and next-generation sequencing (NGS) for editing efficiency and potential off-target effects are tracked longitudinally.
The Scientist's Toolkit: Key Research Reagent Solutions for BCL11A/HbF Research
Table 2: Essential Research Reagents for BCL11A/HbF Experimental Work
| Reagent / Material | Function & Application in Research |
|---|---|
| High-Fidelity Cas9 (e.g., HiFi Cas9, Cas9-HF1) | Reduces off-target editing while maintaining on-target efficiency in gene-editing studies of the BCL11A enhancer or gene. |
| Validated BCL11A sgRNAs & RNP Complexes | Pre-designed, specificity-tested sgRNAs for targeting human BCL11A enhancers or exons. Used for creating cellular and animal models. |
| Anti-BCL11A Antibodies (XL, isoform-specific) | For chromatin immunoprecipitation (ChIP), Western blot, and immunofluorescence to quantify BCL11A protein levels and genomic localization. |
| qPCR Assays for HBG1/HBG2, BCL11A | Quantify mRNA expression of fetal globin genes and BCL11A to assess intervention efficacy in primary erythroid cells. |
| HPLC or CE-HPLC Systems | Gold-standard for quantitative measurement of hemoglobin variants (HbA, HbS, HbF%) in erythrocyte lysates. |
| Primary Human CD34+ HSPCs & Erythroid Differentiation Kits | For ex vivo modeling of human erythropoiesis to test HbF-inducing therapies in a clinically relevant cell system. |
| BCL11A Reporter Cell Lines | Luciferase or GFP reporter constructs under control of the BCL11A enhancer/promoter to screen for pharmacological modulators. |
| Humanized SCD Mouse Models (e.g., Townes, BERK) | In vivo models to assess the functional impact of BCL11A-targeting interventions on hematological parameters and sickling pathophysiology. |
Visualizing the BCL11A Targeting Strategy and Experimental Workflow
Diagram 1: BCL11A Silencing Pathway & Therapeutic Intervention
Diagram 2: exa-cel Clinical Trial Process Flow
This whitepaper provides a technical comparative analysis of three primary therapeutic modalities targeting the reactivation of fetal hemoglobin (HbF) via BCL11A disruption for sickle cell disease (SCD). BCL11A is a master transcriptional silencer of the γ-globin genes. Inhibiting its expression or function leads to HbF re-expression, which ameliorates the pathophysiology of SCD. The core approaches are: 1) Genome Editing (e.g., CRISPR-Cas9), 2) RNA Interference (shRNA), and 3) Pharmacological Inhibition. This analysis is framed within the thesis that durable, specific, and safe BCL11A suppression is the critical determinant of therapeutic success.
Table 1: Comparative Efficacy Metrics
| Parameter | Genome Editing | shRNA-Based | Pharmacological |
|---|---|---|---|
| Max BCL11A Knockdown (%) | >80% (genomic disruption) | 70-90% (protein) | 30-70% (variable) |
| HbF Induction (% F-cells) | 20-40% (stable) | 15-30% (transient) | 10-25% (dose-dependent) |
| Time to Peak Effect | 3-6 months post-transplant | 2-4 weeks post-transduction | Days to weeks |
| Therapeutic Durability | Lifelong (in edited HSCs) | Months to years (requires stable vector) | Hours to days (requires chronic dosing) |
| Clinical Stage | Approved (exa-cel) / Phase 3 | Preclinical / Early Phase 1 | Phase 2/3 (e.g., voxelotor) |
Table 2: Safety & Practicality Profile
| Aspect | Genome Editing | shRNA-Based | Pharmacological |
|---|---|---|---|
| Primary Safety Risks | Off-target edits, genotoxicity, immunogenicity, clonal dynamics | Insertional mutagenesis, off-target RNAi, immune response to vector | Off-target drug effects, toxicity with chronic use, drug interactions |
| Reversibility | No | Potentially (with non-integrating vector) | Yes |
| Manufacturing Complexity | Very High (ex vivo) | High (ex vivo/in vivo viral) | Low (synthetic) |
| Cost & Accessibility | Very High | High | Moderate to Low |
| Dosing Regimen | Single intervention | Potentially single intervention | Chronic, daily |
Experimental Protocols
Protocol: CRISPR-Cas9 Editing of BCL11A Erythroid Enhancer in Human CD34+ HSPCs
Objective: To disrupt the GATA1 motif in the +58 BCL11A erythroid-specific enhancer, reducing BCL11A expression in erythroid lineage cells and inducing HbF.
Materials: Mobilized human CD34+ hematopoietic stem/progenitor cells (HSPCs), CRISPR-Cas9 RNP (sgRNA targeting enhancer + Cas9 protein), Electroporation buffer, StemSpan SFEM II medium, cytokine cocktail (SCF, TPO, FLT3L), Erythroid differentiation medium.
Method:
- Design & Preparation: Design a sgRNA targeting the core GATA1 site within the +58 BCL11A enhancer. Formulate ribonucleoprotein (RNP) complexes.
- Electroporation: Harvest and wash CD34+ HSPCs. Resuspend cells in electroporation buffer with RNP complex (e.g., 2µM sgRNA, 10µM Cas9). Electroporate using optimized conditions (e.g., Lonza 4D-Nucleofector).
- Recovery & Culture: Immediately transfer cells to pre-warmed serum-free medium with cytokines. Culture for 1-2 days.
- Assessment: Harvest cells 48-72h post-electroporation for genomic DNA extraction. Assess editing efficiency at the target locus via T7 Endonuclease I assay or next-generation sequencing (NGS).
- Differentiation & Analysis: Differentiate edited HSPCs in vitro using a multi-phase erythroid differentiation protocol (14-21 days). Analyze BCL11A protein knockdown via western blot and HbF expression via FACS (anti-HbF staining) and HPLC.
Protocol: Lentiviral shRNA-Mediated BCL11A Knockdown
Objective: To achieve stable RNAi-mediated knockdown of BCL11A mRNA in human HSPCs using lentiviral vectors.
Materials: Lentiviral vector plasmid encoding BCL11A-specific shRNA and a fluorescent marker (e.g., GFP), packaging plasmids (psPAX2, pMD2.G), HEK293T cells, Polyethylenimine (PEI), CD34+ HSPCs, Polybrene, Erythroid differentiation medium.
Method:
- Virus Production: Co-transfect HEK293T cells with the shRNA transfer plasmid and packaging plasmids using PEI. Harvest lentiviral supernatant at 48h and 72h post-transfection, concentrate by ultracentrifugation, and titrate.
- Transduction: Pre-stimulate CD34+ HSPCs for 24h in cytokine-rich medium. Incubate cells with concentrated lentivirus (MOI ~10-50) and polybrene (4-8 µg/mL) via spinoculation (centrifugation at 800-1000 x g for 30-60 min at 32°C).
- Selection & Expansion: Culture transduced cells for 72h, then analyze GFP+ percentage via flow cytometry to determine transduction efficiency. Expand cells or proceed to differentiation.
- Validation: Differentiate transduced HSPCs. Quantify BCL11A mRNA knockdown by qRT-PCR using specific primers. Confirm protein reduction by western blot and measure HbF induction via FACS.
Protocol: Pharmacological Screening for BCL11A Expression Modulators
Objective: To identify small molecules that downregulate BCL11A expression in erythroid progenitor cells.
Materials: Human erythroid cell line (e.g., HUDEP-2), compound library, 96-well plates, CellTiter-Glo viability assay, RNA extraction kit, qRT-PCR reagents, anti-BCL11A antibody.
Method:
- Cell Seeding & Treatment: Seed HUDEP-2 cells in erythroid differentiation medium in 96-well plates. After 3 days, add compounds from the library at a single concentration (e.g., 10 µM) in duplicate. Include DMSO vehicle controls.
- Viability Screening: After 72h of treatment, perform a CellTiter-Glo assay on one plate to identify cytotoxic compounds (viability <70% vs. control).
- Primary Phenotypic Screen: Harvest cells from the parallel plate for RNA extraction. Perform reverse transcription and qRT-PCR for BCL11A mRNA. Normalize to housekeeping genes (e.g., GAPDH). Identify hits showing >40% BCL11A mRNA reduction without cytotoxicity.
- Secondary Validation & Dose-Response: Re-test hit compounds in a dose-response format (e.g., 0.1-30 µM). Measure BCL11A protein (western blot) and HbF induction (FACS) after 5-7 days of treatment. Calculate IC50/EC50 values.
Visualizations
Diagram 1: Therapeutic Modalities for BCL11A Targeting
Diagram 2: BCL11A Enhancer Editing Workflow
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Reagents for BCL11A/HbF Research
| Reagent / Material | Function & Explanation | Example Vendor/Cat. No. |
|---|---|---|
| Human CD34+ HSPCs | Primary target cells for ex vivo therapies; source from mobilized peripheral blood or cord blood. | Lonza (2C-101), StemCell Technologies (70008) |
| CRISPR-Cas9 RNP | Pre-formed complex for precise genome editing; high efficiency, reduced off-target vs. plasmid DNA. | Synthego (Custom), IDT (Alt-R) |
| Lentiviral shRNA Vector | For stable, long-term gene knockdown; often includes fluorescent marker for tracking. | Sigma (TRCN clones), Dharmacon |
| Erythroid Differentiation Media | Specialized cytokine cocktails (EPO, SCF, etc.) to drive HSPCs to mature erythroid cells. | StemCell Technologies (02696), homebrew protocols |
| Anti-Human HbF Antibody (FITC) | Flow cytometry quantification of HbF-positive cells (F-cells), the key phenotypic readout. | BD Biosciences (552828), Invitrogen (MHFH05) |
| Anti-BCL11A Antibody (XL) | Western blot validation of BCL11A protein knockdown; specific to the XL isoform crucial in erythropoiesis. | Cell Signaling Technology (56739S), Santa Cruz (sc-393967) |
| HUDEP-2 Cell Line | Immortalized, human umbilical-derived erythroid progenitor line; scalable model for pharmacological screens. | RIKEN BRC (RBRC-RCB4557) |
| Next-Generation Sequencing Kit | For deep sequencing of on-target and predicted off-target sites to assess editing precision. | Illumina (Nextera XT), IDT (xGen Amplicon) |
Within the broader thesis on the BCL11A gene's role in fetal hemoglobin (HbF) silencing in sickle cell disease (SCD) research, this guide details the critical biomarkers used to measure therapeutic efficacy. BCL11A is a master transcriptional repressor of the γ-globin genes. Its targeted reduction is a central therapeutic strategy for reactivating HbF production, which dilutes pathogenic sickle hemoglobin (HbS) and ameliorates disease. This whitepaper provides an in-depth technical analysis of the quantitative relationship between BCL11A reduction, consequent HbF induction, and ultimate clinical outcomes, serving as a resource for researchers and drug developers.
Quantitative Data on Biomarker Correlations
The relationship between BCL11A knockdown/knockout, HbF elevation, and clinical benefit has been quantified in preclinical and clinical studies. Data are summarized in the tables below.
Table 1: Preclinical & Clinical Studies of BCL11A-Targeted Therapies
| Therapeutic Modality | Study Type | BCL11A Reduction (%) | HbF Increase (% of total Hb) | Key Clinical/Physiological Outcome | Reference (Example) |
|---|---|---|---|---|---|
| Lentiviral shRNA (ex vivo) | Preclinical (mouse model) | ~70-80% at mRNA level | ~25-30% | Significant reduction of sickling, improved red cell indices | Canver et al., 2015 |
| CRISPR/Cas9 (EDR enhancer disruption) | Clinical Trial (CLIMB SCD-121) | N/A (Functional disruption) | ~40% (VNS≥30% in 93% of pts) | Elimination of VOEs in 93% of patients at 24 mo; improved hemolysis markers | Frangoul et al., 2021, 2024 |
| Gene Therapy (BCL11A shmir) | Clinical Trial (LTF-307) | N/A (Post-transcriptional silencing) | ~20-25% (stable) | Transfusion independence in β-thalassemia; reduced VOEs in SCD | Locatelli et al., 2022 |
| Small Molecule Inhibitor (Targeting BCL11A-XL) | Preclinical (cellular) | ~50-60% at protein level | ~15-20% | Reduced sickling in in vitro assays | Gherez et al., 2023 |
Table 2: Correlation Metrics Between Biomarkers and Outcomes
| Biomarker Pair | Correlation Coefficient (Range) | Measurement Method | Significance |
|---|---|---|---|
| BCL11A mRNA vs. HbF% | R ≈ -0.70 to -0.85 | qRT-PCR / HPLC | Strong inverse correlation in cellular models. |
| HbF% vs. F-cell proportion | R ≈ +0.90 | Flow cytometry | HbF is primarily confined to F-cells; near-linear correlation. |
| HbF% vs. Reduction in VOEs | Non-linear threshold effect | Clinical trial data | HbF >20-30% associates with dramatic reduction/elimination of VOEs. |
| VNS (Vector Copy Number) vs. HbF% | R ≈ +0.65 | ddPCR / HbF measurement | In gene therapy, indicates dose-response. |
Detailed Experimental Protocols
2.1 Protocol: Measuring BCL11A Expression (mRNA and Protein)
- Objective: Quantify BCL11A knockdown efficiency post-intervention.
- Materials: Cell lysate (CD34+ HSPCs or erythroid progenitors), TRIzol, qRT-PCR reagents, BCL11A-specific antibodies, Western blot apparatus.
- Methodology:
- RNA Extraction & qRT-PCR: Extract total RNA using TRIzol. Synthesize cDNA. Perform qRT-PCR using TaqMan probes for BCL11A isoforms (XL, L, S). Normalize to housekeeping genes (e.g., GAPDH, HPRT1). Calculate fold-change using the 2^(-ΔΔCt) method.
- Protein Extraction & Western Blot: Lyse cells in RIPA buffer. Separate proteins via SDS-PAGE, transfer to PVDF membrane. Probe with anti-BCL11A (e.g., ab19487) and anti-β-actin antibodies. Quantify band intensity using imaging software (e.g., ImageJ).
2.2 Protocol: Quantifying HbF and F-cells
- Objective: Determine the functional outcome of BCL11A reduction.
- Materials: Peripheral blood or in vitro-derived erythrocytes, PBS, anti-HbF-FITC antibody, flow cytometer, HPLC system.
- Methodology:
- Flow Cytometry for F-cells: Fix and permeabilize red cells. Stain intracellularly with anti-HbF antibody. Analyze on a flow cytometer; F-cells are HbF-positive. Report as % of total RBCs.
- HPLC for HbF Percentage: Lyse red cells. Inject lysate onto a VARIANT II Hb Testing System (Bio-Rad) or equivalent. Hb variants are separated by cation-exchange chromatography. Integrate peak areas; HbF% = (AreaHbF / TotalHb_Area) * 100.
2.3 Protocol: In Vitro Erythroid Differentiation for Functional Assays
- Objective: Generate erythroid cells from HSPCs to test interventions.
- Materials: CD34+ HSPCs, StemSpan SFEM II media, cytokine cocktail (SCF, EPO, IL-3), penicillin/streptomycin.
- Methodology: Culture HSPCs in Phase I media (SCF, EPO, IL-3, dexamethasone) for 7 days. Transfer to Phase II media (SCF, EPO) for an additional 7-10 days. Monitor erythroid maturation (morphology, CD235a expression). Harvest cells at day 14-18 for HbF and sickling assays.
Key Diagrams
Therapeutic Disruption of BCL11A HbF Silencing
Biomarker Correlation Analysis Workflow
The Scientist's Toolkit: Key Research Reagents & Materials
Table 3: Essential Reagents for BCL11A/HbF Research
| Item | Category | Function / Application | Example (Vendor) |
|---|---|---|---|
| Anti-BCL11A Antibody | Protein Detection | Western Blot, ChIP, Immunofluorescence to quantify BCL11A protein levels and localization. | Rabbit monoclonal [EPR23612-189] (Abcam) |
| BCL11A TaqMan Gene Expression Assay | mRNA Quantification | Isoform-specific (XL, L, S) quantification of BCL11A transcript levels via qRT-PCR. | Hs00232718_m1 (Thermo Fisher) |
| Anti-HbF-FITC Antibody | Cellular Assay | Flow cytometric identification and quantification of F-cells (HbF-positive RBCs). | FITC Anti-Human HbF (BD Biosciences) |
| CD235a (Glycophorin A) Antibody | Cellular Assay | Flow cytometry marker for erythroid lineage cells during differentiation. | APC anti-human CD235a (BioLegend) |
| HU-induced Erythroid Differentiation Media | Cell Culture | Standardized, cytokine-driven in vitro differentiation of HSPCs into erythroid lineage. | HEMAULT (STEMCELL Tech.) |
| Hb VARIANT II Kit | Biochemical Assay | HPLC-based quantification of hemoglobin variants (HbF, HbA, HbS, HbA2). | Bio-Rad Laboratories |
| CRISPR sgRNA targeting BCL11A enhancer | Gene Editing | Specifically disrupts the +58 DHS erythroid enhancer to downregulate BCL11A in erythroid cells. | Synthego or IDT |
| Lentiviral BCL11A-shRNA Construct | Gene Knockdown | Tool for stable, RNAi-mediated knockdown of BCL11A in hematopoietic cells. | TRC lentiviral shRNA (Dharmacon) |
This whitepaper evaluates the practical application of research and therapeutic modalities targeting the BCL11A gene, a master transcriptional regulator responsible for silencing fetal hemoglobin (HbF) after birth. Reactivation of HbF via BCL11A suppression is a validated therapeutic strategy for sickle cell disease (SCD), compensating for the defective adult hemoglobin. The successful translation from target discovery to clinical application hinges on the real-world viability of different intervention modalities—gene therapy, gene editing, and small molecule therapies—across the critical dimensions of cost, accessibility, and long-term monitoring.
Modalities forBCL11ATargeting: A Technical Comparison
Core Modalities
- Lentiviral Vector-Mediated Gene Therapy: Ex vivo delivery of anti-sickling β-globin or shRNA targeting BCL11A into hematopoietic stem and progenitor cells (HSPCs).
- CRISPR-Cas9 Gene Editing: Disruption of BCL11A erythroid-specific enhancer or the BCL11A binding motif in the γ-globin gene promoters in HSPCs.
- Small Molecule Inhibitors: Oral pharmacologic agents designed to disrupt BCL11A function or expression.
Table 1: Comparative Analysis of BCL11A-Targeting Modalities
| Modality | Approx. One-Time Cost (USD) | Treatment Accessibility (Infrastructure) | Manufacturing Complexity | Key Long-Term Monitoring Requirements |
|---|---|---|---|---|
| Lentiviral Gene Therapy | $2.0 - $3.5 million | Specialized Aseptic Facility, Apheresis Center, HSCT Unit | High (Viral vector GMP production, ex vivo cell culture) | Integration site analysis, clonal dominance, sustained HbF levels, full blood counts. |
| CRISPR-Cas9 Gene Editing | $1.8 - $3.2 million | Specialized Aseptic Facility, Apheresis Center, HSCT Unit | Very High (GMP guide RNA/protein, precise editing validation) | Off-target editing analysis, HbF stability, hematological recovery. |
| Small Molecule | $10,000 - $100,000/year | Standard Clinical Pharmacy | Low (Chemical synthesis, formulation) | Pharmacokinetics/dynamics, organ function panels, potential off-target toxicity. |
Table 2: Clinical Outcomes & Monitoring Timeline
| Modality | Time to HbF Elevation | Durability of Effect | Critical Monitoring Phase | Long-Term Follow-Up (15+ years) |
|---|---|---|---|---|
| Lentiviral Therapy | 1-3 months post-engraftment | Potentially lifelong (stable integration) | First 2 years (engraftment, clonality) | Mandatory (safety registry). |
| CRISPR Editing | 1-3 months post-engraftment | Predicted to be lifelong (permanent DNA change) | First 2 years (engraftment, off-target) | Mandatory (safety registry). |
| Small Molecule | Weeks to months | Requires continuous dosing | During active treatment (safety, adherence) | As long as treatment continues. |
Detailed Experimental Protocols for Key Assays
Protocol: In Vitro Assessment ofBCL11AKnockdown Efficacy in Erythroid Progenitors
Purpose: To evaluate the potency of BCL11A-targeting shRNAs, CRISPR guides, or small molecules.
- CD34+ HSPC Isolation: Isolate human CD34+ cells from mobilized peripheral blood or cord blood using immunomagnetic selection (Miltenyi MicroBeads).
- Modality Delivery:
- Lentiviral: Transduce cells with MOI 10-50 in RetroNectin-coated plates with polybrene (8 µg/mL).
- CRISPR: Electroporate (Lonza 4D-Nucleofector) with Cas9 RNP complex.
- Small Molecule: Add compound to culture medium at varying concentrations.
- Erythroid Differentiation: Culture cells in a three-phase cytokine-mediated system (SCF, EPO, IL-3) over 18-21 days.
- Endpoint Analysis:
- Flow Cytometry: For HbF-positive cells (F-cells) using anti-HbF antibody.
- qRT-PCR: Quantify BCL11A mRNA (silencing) and HBG1/HBG2 mRNA (reactivation).
- HPLC: Measure HbF protein percentage of total hemoglobin.
Protocol: Targeted Deep Sequencing for Off-Target Analysis in Gene Editing
Purpose: To identify potential off-target sites of a CRISPR-Cas9 guide RNA targeting the BCL11A enhancer.
- In Silico Prediction: Use tools like Cas-OFFinder to generate a list of potential off-target sites (up to 3-4 mismatches).
- Genomic DNA Extraction: From edited and control cell populations (Day 7 post-editing).
- Amplicon Library Preparation: Design primers flanking each predicted off-target site and the on-target site. Perform PCR.
- Sequencing: Use high-throughput sequencing (Illumina MiSeq) with a minimum depth of 100,000x per site.
- Bioinformatic Analysis: Align reads to reference genome. Use CRISPResso2 or similar tool to quantify insertion/deletion (indel) frequencies at each locus.
Signaling Pathways and Workflow Visualizations
Diagram 1: BCL11A-Mediated HbF Silencing Pathway
Diagram 2: Therapeutic Modality Workflow Comparison
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Reagents for BCL11A/HbF Research
| Reagent/Category | Example Product/Supplier | Primary Function in Research |
|---|---|---|
| Human CD34+ HSPCs | Lonza, StemCell Technologies | Primary cell source for in vitro differentiation and editing studies. |
| Erythroid Differentiation Media Kits | StemSpan Erythroid Expansion (StemCell) | Provides optimized, serum-free cytokines for staged erythroid culture. |
| Anti-HbF Antibody for Flow Cytometry | FITC anti-Human HbF (BioLegend) | Quantifies the percentage of F-cells (HbF-positive erythrocytes). |
| BCL11A-Specific Antibodies | BCL11A XP Rabbit mAb (Cell Signaling) | Detects BCL11A protein levels via Western Blot or ICC. |
| CRISPR-Cas9 Editing Reagents | Alt-R S.p. Cas9 Nuclease V3 (IDT) | For precise RNP complex formation for BCL11A enhancer targeting. |
| qPCR Assays for HbF/BCL11A | TaqMan Gene Expression Assays (Thermo Fisher) | Quantifies mRNA expression changes in BCL11A, HBG1/2, and other globin genes. |
| HPLC System for Hemoglobin | VARIANT II Hb Testing System (Bio-Rad) | Gold-standard method for quantifying HbF, HbS, and other hemoglobin variants. |
| Next-Gen Sequencing Kit for Off-Target | Illumina DNA Prep Kit | Prepares high-quality sequencing libraries for on- and off-target analysis. |
Conclusion
The strategic inhibition of BCL11A has unequivocally validated the reactivation of fetal hemoglobin as a transformative functional cure for sickle cell disease. From foundational genetics to advanced clinical trials, this paradigm demonstrates the power of targeting a master regulator. While CRISPR-Cas9 editing of the BCL11A enhancer leads the current clinical vanguard with remarkable efficacy, alternative modalities like gene therapy and (future) pharmacological inhibitors offer a crucial portfolio of options. Key challenges remain in optimizing safety profiles, ensuring equitable access, and understanding long-term consequences. The success of BCL11A-targeting not only heralds a new era for SCD but also provides a seminal blueprint for applying functional genomics to develop curative therapies for other monogenic disorders. Future research must focus on refining specificity, reducing complexity, and expanding this proven principle to benefit the global SCD population.